Abstract
Background
Age-related macular degeneration (AMD) is a major cause of irreversible blindness among elderly people in developed countries. Many studies suggested that age-related maculopathy susceptibility 2 (ARMS2) is the second major susceptibility gene for AMD. Increasing evidence was found recently that inflammatory processes and oxidative stress may contribute to the pathogenesis of AMD. Meanwhile, the mechanisms underlying the contributions of ARMS2 to the pathogenesis of AMD remain unclear. The purpose of the current study was to elucidate the relationship between the ARMS2 gene and proinflammatory mediators, for further assessment of the associated biologic effects.
Methods
siRNA was used to knock down ARMS2 mRNA, and Western blotting and reverse real-time PCR were used to detect the effect of siRNA on the expression of ARMS2 in ARPE-19 cells. The expressions of C3, C5, IL-6, IL-8, and TNF-α after si-RNA knockdown were evaluated by SYBR Green I real-time PCR and ELISA.
Results
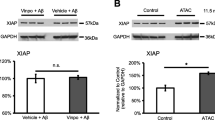
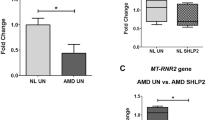
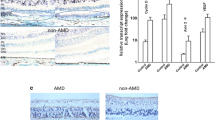
Transcription accumulative indexes (TAI = 2−delta delta CT) of ARMS2 by real-time PCR revealed that the transfection rate in the positive control group was 72.0 ± 2.07 % (P < 0.01). The ratio of absorbance values (by Western blotting) of AMRS2 to β-actin was 0.85 ± 0.122, 0.87 ± 0.143, and 0.61 ± 0.240 in the blank control group, scrambled ARMS2-siRNA group, and ARMS2-siRNA group respectively (F = 42.5, P < 0.01). The secreted protein levels of C3, C5, IL-6, IL-8, and TNF-α were found by ELISA to be reduced by 34.24 ± 1.81 %, 37.15 ± 2.02 %, 35.11 ± 1.75 %, 30.11 ± 2.19 %, and 34.33 ± 2.18 % respectively, in the siRNA-ARMS2 group (P < 0.05). Compared with the blank control group, reduced TAI of C3, C5, IL-6, IL-8, and TNF-α were detected by real-time PCR in the ARMS2-siRNA group.
Conclusion
This study produced evidence supporting the notion that the ARMS2 risk allele for AMD is linked directly or indirectly to proinflammatory mediators. More importantly, our data indicate that the change in ARMS2 may affect C3, C5, IL-6, IL-8, and TNF-α levels, and this may be one of the mechanisms of AMD development.



Similar content being viewed by others
References
Jager RD, Mieler WF, Miller JW (2008) Age-related macular degeneration. N Engl J Med 358:2606–2617
Kikuchi M, Nakamura M, Ishikawa K, Suzuki T, Nishihara H, Yamakoshi T, Nishio K, Taki K, Niwa T, Hamajima N, Terasaki H (2007) Elevated C-reactive protein levels in patients with polypoidal choroidal vasculopathy and patients with neovascular age-related macular degeneration. Ophthalmology 114:1722–1727
Oshima Y, Ishibashi T, Murata T, Tahara Y, Kiyohara Y, Kubota T (2001) Prevalence of age related maculopathy in a representative Japanese population: the Hisayama study. Br J Ophthalmol 85:1153–1157
Klein R, Peto T, Bird A, Vannewkirk MR (2004) The epidemiology of age-related macular degeneration. Am J Ophthalmol 137:486–495
Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP (2003) Age related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 48:257–293
Donoso LA, Kim D, Frost A, Callahan A, Hageman G (2006) The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 51:137–152
Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL (2008) Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med 14:194–198
Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH (2005) Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet 14:3227–3236
Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB (2005) Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet 77:389–407
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J (2005) Complement factor H polymorphism in age-related macular degeneration. Science 308:385–389
Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA (2005) Complement factor H polymorphism and age related macular degeneration. Science 308:421–424
Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA (2005) Complement factor H variant increases the risk of age related macular degeneration. Science 308:419–421
Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT (2007) Genetic Factors in AMD Study Group. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med 357:553–561
Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, AMD Genetics Clinical Study Group, Hageman GS, Dean M, Allikmets R (2007) Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet 38:458–462
Yasuma TR, Nakamura M, Nishiguchi KM, Kikuchi M, Kaneko H, Niwa T, Hamajima N, Terasaki H (2010) Elevated C-reactive protein levels and ARMS2/HTRA1 gene variants in subjects without age-related macular degeneration. Mol Vis 16:2923–2930
Seddon JM, George S, Rosner B, Rifai N (2005) Prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol 123:774–782
Baas DC, Ho L, Ennis S, Merriam JE, Tanck MW, Uitterlinden AG, de Jong PT, Cree AJ, Griffiths HL, Rivadeneira F, Hofman A, van Duijn C, Smith RT, Barile GR, Gorgels TG, Vingerling JR, Klaver CC, Lotery AJ, Allikmets R, Bergen AA (2010) The complement component 5 gene and age-related macular degeneration. Ophthalmology 117:500–511
Bessho H, Honda S, Kondo N, Negi A (2011) The association of age-related maculopathy susceptibility 2 polymorphisms with phenotype in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Mol Vis 17:977–982
Fritsche LG, Loenhardt T, Janssen A, Fisher SA, Rivera A, Keilhauer CN, Weber BH (2008) Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet 40:892–896
Xu YT, Wang Y, Chen P, Xu HF (2012) Age-related maculopathy susceptibility 2 participates in the phagocytosis functions of the retinal pigment epithelium. Int J Ophthalmol 5:125–132
Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A (2007) A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci USA 104:16227–16232
Wang G, Spencer KL, Scott WK, Whitehead P, Court BL, Ayala-Haedo J, Mayo P, Schwartz SG, Kovach JL, Gallins P, Polk M, Agarwal A, Postel EA, Haines JL, Pericak-Vance MA (2010) Analysis of the indel at the ARMS2 3’UTR in age-related macular degeneration. Hum Genet 127:595–602
Wang G, Spencer KL, Court BL, Olson LM, Scott WK, Haines JL, Pericak-Vance MA (2009) Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria. Invest Ophthalmol Vis Sci 50:3084–3090
Kanda A, Stambolian D, Chen W, Curcio CA, Abecasis GR, Swaroop A (2010) Age-related macular degeneration-associated variants at chromosome 10q26 do not significantly alter ARMS2 and HTRA1 transcript levels in the human retina. Mol Vis 16:1317–1323
Yang Z, Tong Z, Chen Y, Zeng J, Lu F, Sun X, Zhao C, Wang K, Davey L, Chen H, London N, Muramatsu D, Salasar F, Carmona R, Kasuga D, Wang X, Bedell M, Dixie M, Zhao P, Yang R, Gibbs D, Liu X, Li Y, Li C, Campochiaro B, Constantine R, Zack DJ, Campochiaro P, Fu Y, Li DY, Katsanis N, Zhang K (2010) Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS Genet 6:e1000836
Gotoh N, Yamada R, Nakanishi H, Saito M, Iida T, Matsuda F, Yoshimura N (2008) Correlation between CFH Y402H and HTRA1 rs11200638 genotype to typical exudative age-related macular degeneration and polypoidal choroidal vasculopathy phenotype in the Japanese population. Clin Exp Ophthalmol 36:437–442
Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J (2006) HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 314:989–992
Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K (2006) A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 314:992–993
Chen H, Yang Z, Gibbs D, Yang X, Hau V, Zhao P, Ma X, Zeng J, Luo L, Pearson E, Constantine R, Kaminoh Y, Harmon J, Tong Z, Stratton CA, Cameron DJ, Tang S, Zhang K (2008) Association of HTRA1 polymorphism and bilaterality in advanced age-related macular degeneration. Vision Res 48:690–694
Hasegawa E, Oshima Y, Takeda A, Saeki K, Yoshida H, Sonoda KH, Ishibashi T (2012) IL-27 inhibits pathophysiological intraocular neovascularization due to laser burn. J Leukoc Biol 91:267–273
Matsumura N, Kamei M, Tsujikawa M, Suzuki M, Xie P, Nishida K (2012) Low-dose lipopolysaccharide pretreatment suppresses choroidal neovascularization via IL-10 induction. PLoS One 7:e39890
Patel AK, Hackam AS (2012) Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol 54:122–131
Acknowledgments
The authors thank Ms Ping Lin for her editorial assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, F., Zhang, M., Xu, Y. et al. ARMS2 interference leads to decrease of proinflammatory mediators. Graefes Arch Clin Exp Ophthalmol 251, 2539–2544 (2013). https://doi.org/10.1007/s00417-013-2442-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2442-0