Abstract
Background
Choroidal neovascularisation (CNV) as a feature of exudative age-related macular degeneration (AMD) is partially regulated by retinal pigment epithelium (RPE). In this study, the effect of combinatory anti-angiogenic treatment was evaluated using a novel in vitro assay of RPE-induced angiogenesis.
Methods
RPE isolated from surgically excised CNV-membranes (CNV-RPE) was used to stimulate sprouting of endothelial cell (EC) spheroids in a 3D collagen matrix. The anti-angiogenic effect of solitary anti-VEGF antibodies (bevacizumab) was compared to a combinatory treatment with anti-VEGF and anti-FGF2 antibodies.
Results
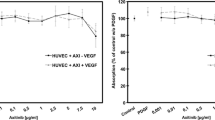
Anti-VEGF treatment inactivated all RPE-derived VEGF but was unable to fully inhibit EC sprouting induced by CNV-RPE. Combined anti-VEGF/anti-FGF treatment inactivated both growth factors and reduced EC sprouting significantly.
Conclusions
RPE from CNV patients expresses angiogenic growth factors that act in part independently of VEGF. Targeted combinatory therapy can be superior to solitary anti-VEGF therapy. One possible candidate for combinatory therapy is FGF2.




Similar content being viewed by others
References
Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870. doi:10.1097/01.iae.0000242842.14624.e7
Miller H, Miller B, Ryan SJ (1986) The role of retinal pigment epithelium in the involution of subretinal neovascularization. Invest Ophthalmol Vis Sci 27:1644–1652
Sakamoto T, Sakamoto H, Hinton DR, Spee C, Ishibashi T, Ryan SJ (1995) In vitro studies of human choroidal endothelial cells. Curr Eye Res 14:621–627. doi:10.3109/02713689508998488
Cai J, Nelson KC, Wu M, Sternberg P Jr, Jones DP (2000) Oxidative damage and protection of the RPE. Prog Retin Eye Res 19:205–221. doi:10.1016/S1350-9462(99)00009-9
Ng EW, Adamis AP (2005) Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol 40:352–368
Ni M, Holland M, Jarstadmarken H, De Vries G (2005) Time-course of experimental choroidal neovascularization in Dutch-Belted rabbit: clinical and histological evaluation. Exp Eye Res 81:286–297
Gibran SK, Sachdev A, Stappler T, Newsome R, Wong D, Hiscott P (2007) Histological findings of a choroidal neovascular membrane removed at the time of macular translocation in a patient previously treated with intravitreal bevacizumab treatment (Avastin). Br J Ophthalmol 91:602–604. doi:10.1136/bjo.2006.108795
Casaroli-Marano RP, Pagan R, Vilaro S (1999) Epithelial-mesenchymal transition in proliferative vitreoretinopathy: intermediate filament protein expression in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 40:2062–2072
Grisanti S, Guidry C (1995) Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci 36:391–405
Schlunck G, Martin G, Agostini HT, Camatta G, Hansen LL (2002) Cultivation of retinal pigment epithelial cells from human choroidal neovascular membranes in age related macular degeneration. Exp Eye Res 74:571–576. doi:10.1006/exer.2001.1148
Stahl A, Paschek L, Martin G, Gross NJ, Feltgen N, Hansen LL, Agostini HT (2008) Rapamycin reduces VEGF expression in retinal pigment epithelium (RPE) and inhibits RPE-induced sprouting angiogenesis in vitro. FEBS Lett 582:3097–3102. doi:10.1016/j.febslet.2008.08.005
Korff T, Augustin HG (1998) Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol 143:1341–1352. doi:10.1083/jcb.143.5.1341
Korff T, Augustin HG (1999) Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci 112(Pt 19):3249–3258
Wenger A, Stahl A, Weber H, Finkenzeller G, Augustin HG, Stark GB, Kneser U (2004) Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng 10:1536–1547
Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT (1997) Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 81:154–162. doi:10.1136/bjo.81.2.154
Otani A, Takagi H, Oh H, Koyama S, Matsumura M, Honda Y (1999) Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Invest Ophthalmol Vis Sci 40:1912–1920
Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO (1999) Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol 155:421–428
Ishigooka H, Aotaki-Keen AE, Hjelmeland LM (1992) Subcellular localization of bFGF in human retinal pigment epithelium in vitro. Exp Eye Res 55:203–214. doi:10.1016/0014-4835(92)90184-T
Amin R, Puklin JE, Frank RN (1994) Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci 35:3178–3188
Reddy VM, Zamora RL, Kaplan HJ (1995) Distribution of growth factors in subfoveal neovascular membranes in age-related macular degeneration and presumed ocular histoplasmosis syndrome. Am J Ophthalmol 120:291–301
Martin G, Schlunck G, Hansen LL, Agostini HT (2004) Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 242:321–326. doi:10.1007/s00417-003-0838-y
Chen CY, Wong TY, Heriot WJ (2007) Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration: a short-term study. Am J Ophthalmol 143:510–512. doi:10.1016/j.ajo.2006.10.004
Giansanti F, Virgili G, Bini A, Rapizzi E, Giacomelli G, Donati MC, Verdina T, Menchini U (2007) Intravitreal bevacizumab therapy for choroidal neovascularization secondary to age-related macular degeneration: 6-month results of an open-label uncontrolled clinical study. Eur J Ophthalmol 17:230–237
Emerson MV, Lauer AK, Flaxel CJ, Wilson DJ, Francis PJ, Stout JT, Emerson GG, Schlesinger TK, Nolte SK, Klein ML (2007) Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina 27:439–444. doi:10.1097/IAE.0b013e31804b3e15
McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA (2002) Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci 43:1986–1993
Rosenthal R, Strauss O (2003) Investigations of RPE cells of choriodal neovascular membranes from patients with age-related macula degeneration. Adv Exp Med Biol 533:107–113
Rosenthal R, Heimann H, Agostini H, Martin G, Hansen LL, Strauss O (2007) Ca2+ channels in retinal pigment epithelial cells regulate vascular endothelial growth factor secretion rates in health and disease. Mol Vis 13:443–456
Hunt RC, Davis AA (1990) Altered expression of keratin and vimentin in human retinal pigment epithelial cells in vivo and in vitro. J Cell Physiol 145:187–199. doi:10.1002/jcp.1041450202
Stahl A, Wu X, Wenger A, Klagsbrun M, Kurschat P (2005) Endothelial progenitor cell sprouting in spheroid cultures is resistant to inhibition by osteoblasts: a model for bone replacement grafts. FEBS Lett 579:5338–5342. doi:10.1016/j.febslet.2005.09.005
Korff T, Kimmina S, Martiny-Baron G, Augustin HG (2001) Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J 15:447–457. doi:10.1096/fj.00-0139com
Takeda Y, Kazarov AR, Butterfield CE, Hopkins BD, Benjamin LE, Kaipainen A, Hemler ME (2007) Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood 109:1524–1532. doi:10.1182/blood-2006-08-041970
Geisen P, McColm JR, Hartnett ME (2006) Choroidal endothelial cells transmigrate across the retinal pigment epithelium but do not proliferate in response to soluble vascular endothelial growth factor. Exp Eye Res 82:608–619. doi:10.1016/j.exer.2005.08.021
Dorrell MI, Aguilar E, Scheppke L, Barnett FH, Friedlander M (2007) Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci USA 104:967–972. doi:10.1073/pnas.0607542104
Castellon R, Hamdi HK, Sacerio I, Aoki AM, Kenney MC, Ljubimov AV (2002) Effects of angiogenic growth factor combinations on retinal endothelial cells. Exp Eye Res 74:523–535. doi:10.1006/exer.2001.1161
Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL (1997) Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 57:963–969
Spaide RF (2006) Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol 141:149–156. doi:10.1016/j.ajo.2005.07.025
Stavri GT, Zachary IC, Baskerville PA, Martin JF, Erusalimsky JD (1995) Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells. Synergistic interaction with hypoxia. Circulation 92:11–14
Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P (1998) Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol 141:1659–1673. doi:10.1083/jcb.141.7.1659
Zubilewicz A, Hecquet C, Jeanny JC, Soubrane G, Courtois Y, Mascarelli F (2001) Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells: a comparative study with VEGF. Oncogene 20:1403–1413. doi:10.1038/sj.onc.1204231
Im E, Kazlauskas A (2006) Regulating angiogenesis at the level of PtdIns-4,5-P2. EMBO J 25:2075–2082. doi:10.1038/sj.emboj.7601100
Acknowledgments
The authors wish to thank Beatrix Flügel, Renate Buchen and Anne Mattes for excellent technical support. This work was supported by funding from the Forschungskommission Freiburg (AS).
Author information
Authors and Affiliations
Corresponding author
Additional information
None of the authors has financial relationships with companies or organisations mentioned in the study.
Rights and permissions
About this article
Cite this article
Stahl, A., Paschek, L., Martin, G. et al. Combinatory inhibition of VEGF and FGF2 is superior to solitary VEGF inhibition in an in vitro model of RPE-induced angiogenesis. Graefes Arch Clin Exp Ophthalmol 247, 767–773 (2009). https://doi.org/10.1007/s00417-009-1058-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1058-x