Abstract
Background
Intravitreal triamcinolone acetonide (TA) has been widely used as a therapeutic method for many ocular diseases, but a consensus on an appropriate safe therapeutic window of dosage for intravitreal injection, and whether vehicle of TA should be reduced or eliminated, has not yet been reached. The aim of this article is to investigate these issues.
Methods
Forty New Zealand white rabbits were divided into four experimental groups and one control group. Four or 25 mg TA, with vehicle either reduced or not, was injected into the vitreous cavity of rabbits in experimental groups. Rabbits in the control group received 0.2 ml intravitreal sterile saline solution. Intraocular pressures (IOP) were measured by a Tonopen tonometer. Values of lens density were measured by a Pentacam system. Soluble protein, total antioxidation capacity, reduced glutathione (GSH), glutathion peroxidase (GSH-px), and superoxide dismutase (SOD) in lens were measured by specific kits. ERG and pathological examinations, including light and electron microscopy of the retina, were also performed.
Results
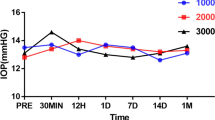
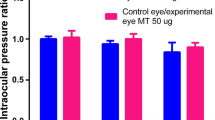
Elevation of IOP was noted in all experimental groups after intravitreal TA (P<0.01, paired t-test). Significant increase of lens density was noticed at 1 week after intravitreal TA in the 25 mg vehicle-containing group (P<0.0001, paired t-test). Significant loss of GSH-px activity was noticed at the end of the study (P<0.05, paired t-test), while SOD activity increased (P<0.05, paired t-test). Amplitudes of ERG waves declined significantly in vehicle-containing groups (P<0.01, paired t-test) at the end of the study. Pathological examination showed obvious retinal toxicity in vehicle-containing groups.
Conclusions
Vehicle of TA should be eliminated or reduced before intravitreal injection to avoid potential retinal toxicity and transient increase in lens density.





Similar content being viewed by others
References
Bednarek J, Wysocki H, Sowinski J (2004) Peripheral parameters of oxidative stress in patients with infiltrative Graves’ ophthalmopathy treated with corticosteroids. Immunol Lett 93(2–3):227–232
Bucher RS, Johnson MW (2005) Microbiologic studies of multiple-dose containers of triamcinolone acetonide and lidocaine hydrochloride. Retina 25(3):269–271
Bynoe LA, Weiss JN (2003) Retinal endovascular surgery and intravitreal triamcinolone acetonide for central vein occlusion in young adults. Am J Ophthalmol 135(3):382–384
Ciulla TA, Criswell MH, Danis RP, Fronheiser M, Yuan P, Cox TA, Csaky KG, Robinson MR (2003) Choroidal neovascular membrane inhibition in a laser treated rat model with intraocular sustained release triamcinolone acetonide microimplants. Br J Ophthalmol 87(8):1032–1037
Danis RP, Ciulla TA, Pratt LM, Anliker W (2000) Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina 20(3):244–250
Dickerson JE Jr, Dotzel E, Clark AF (1997) Steroid-induced cataract: new perspective from in vitro and lens culture studies. Exp Eye Res 65(4):507–516
Fydryk J, Jacobson E, Kurzawska O, Malecka G, Gonet B, Urasinski T, Brodkiewicz A, Bukowska H (1998) Antioxidant status of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 12(9):751–754
Garcia-Arumi J, Boixadera A, Giralt J, Martinez-Castillo V, Gomez-Ulla F, Corcostegui B, Garcia-Arumi E (2005) Comparison of different techniques for purification of triamcinolone acetonide suspension for intravitreal use. Br J Ophthalmol 89(9):1112–1114
Gerometta R, Podos SM, Candia OA, Wu B, Malgor LA, Mittag T, Danias J (2004) Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol 122(10):1492–1497
Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM (2005) Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology 112(1):139–143
Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, Mitchell P, Zhu M, Hunyor AB (2004) Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol 122(3):336–340
Hanta I, Kuleci S, Canacankatan N, Kocabas A (2003) The oxidant-antioxidant balance in mild asthmatic patients. Lung 181(6):347–352
Hernaez-Ortega MC, Soto-Pedre E (2004) A simple and rapid method for purification of triamcinolone acetonide suspension for intravitreal injection. Ophthalmic Surg Lasers Imaging 35(4):350–351
Hida T, Chandler D, Arena JE, Machemer R (1986) Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. Am J Ophthalmol 101(2):190–195
Hui YN, Hu D (1999) Prevention of experimental proliferative vitreoretinopathy with daunomycin and triamcinolone based on the time course of the disease. Graefes Arch Clin Exp Ophthalmol 237(7):601–605
Jonas JB, Degenring R, Kreissig I, Akkoyun I (2004) Safety of intravitreal high-dose reinjections of triamcinolone acetonide. Am J Ophthalmol 138(6):1054–1055
Jonas JB, Hayler JK, Panda-Jonas S (2000) Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol 84(9):1064–1067
Jonas JB, Hayler JK, Sofker A, Panda-Jonas S (2001) Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol 131(4):468–471
Jonas JB, Hayler JK, Sofker A, Panda-Jonas S (2001) Regression of neovascular iris vessels by intravitreal injection of crystalline cortisone. J Glaucoma 10(4):284–287
Jonas JB, Kreissig I, Sofker A, Degenring RF (2003) Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 121(1):57–61
Kivilcim M, Peyman GA, El-Dessouky ES, Kazi AA, Cheema R, Hegazy H (2000) Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. Ophthalmic Surg Lasers 31(6):474–478
Kosano H, Nishigori H (2002) Steroid-induced cataract: other than in the whole animal system, in the lens culture system, androgens, estrogens and progestins as well as glucocorticoids produce a loss of transparency of the lens. Dev Ophthalmol 35:161–168
Lee JW, Iwatsuru M, Nishigori H (1995) Glucocorticoid-induced cataract of developing chick embryo as a screening model for anticataract agents. J Ocul Pharmacol Ther 11(4):533–541
McCuen BW 2nd, Bessler M, Tano Y, Chandler D, Machemer R (1981) The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol 91(6):785–788
Mohan R, Muralidharan AR (1989) Steroid induced glaucoma and cataract. Ind J Ophthalmol 37(1):13–16
Moshfeghi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinesterra JP, Kaiser RS, Bakri SJ, Maturi RK, Belmont J, Beer PM, Murray TG, Quiroz-Mercado H, Mieler WF (2003) Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 136(5):791–796
Nishimura A, Kobayashi A, Segawa Y, Sakurai M, Shirao E, Shirao Y, Sugiyama K (2003) Isolating triamcinolone acetonide particles for intravitreal use with a porous membrane filter. Retina 23(6):777–779
Park CH, Jaffe GJ, Fekrat S (2003) Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol 136(3):419–425
Patteau F, Bourcier T, Naacke H, Allahdadi H, Borderie V, Laroche L (2003) [Posterior subcapsular cataract in a case of ulcerative colitis treated with corticosteroid enema]. J Fr Ophtalmol 26(8):834–836
Pescosolido N, Miccheli A, Manetti C, Iannetti GD, Feher J, Cavallotti C (2001) Metabolic changes in rabbit lens induced by treatment with dexamethasone. Ophthalmic Res 33(2):68–74
Rechtman E, Allen VD, Danis RP, Pratt LM, Harris A, Speicher MA (2003) Intravitreal triamcinolone for choroidal neovascularization in ocular histoplasmosis syndrome. Am J Ophthalmol 136(4):739–741
Singh IP, Ahmad SI, Yeh D, Challa P, Herndon LW, Allingham RR, Lee PP (2004) Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol 138(2):286–287
Spandau UH, Derse M, Schmitz-Valckenberg P, Papoulis C, Sagstetter BU, Stiefvater K, Jonas JB (2005) Triamcinolone acetonide concentration after filtration of the solvent agent. Am J Ophthalmol 139(4):712–713
Sutter FK, Gillies MC (2003) Pseudo-endophthalmitis after intravitreal injection of triamcinolone. Br J Ophthalmol 87(8):972–974
Sutter FK, Simpson JM, Gillies MC (2004) Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology 111(11):2044–2049
Weinreb RN, Bloom E, Baxter JD, Alvarado J, Lan N, O’Donnell J, Polansky JR (1981) Detection of glucocorticoid receptors in cultured human trabecular cells. Invest Ophthalmol Vis Sci 21(3):403–407
Wenk EJ, Hernandez MR, Weinstein BI, Gordon GG, Dunn MW, Southren AL (1982) Glucocorticoid receptor binding in bovine lens. Invest Ophthalmol Vis Sci 22(5):599–605
Wingate RJ, Beaumont PE (1999) Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthalmol 27(6):431–432
Wordinger RJ, Clark AF (1999) Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res 18(5):629–667
Yeung CK, Chan KP, Chan CK, Pang CP, Lam DS (2004) Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: comparison with dexamethasone and hydrocortisone. Jpn J Ophthalmol 48(3):236–242
Yeung CK, Chan KP, Chiang SW, Pang CP, Lam DS (2003) The toxic and stress responses of cultured human retinal pigment epithelium (ARPE19) and human glial cells (SVG) in the presence of triamcinolone. Invest Ophthalmol Vis Sci 44(12):5293–5300
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the 985 Fund of Peking University, Beijing.
The authors have no financial interest in any instruments or drugs mentioned in this study.
Rights and permissions
About this article
Cite this article
Kai, W., Yanrong, J. & Xiaoxin, L. Vehicle of triamcinolone acetonide is associated with retinal toxicity and transient increase of lens density. Graefe's Arch Clin Exp Ophthalmo 244, 1152–1159 (2006). https://doi.org/10.1007/s00417-005-0251-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0251-9