Abstract
Background
Past evidence shows that changes in functional brain connectivity in multiple resting-state networks occur in cognitively healthy individuals who have non-modifiable risk factors for Alzheimer’s Disease. Here, we aimed to investigate how those changes differ in early adulthood and how they might relate to cognition.
Methods
We investigated the effects of genetic risk factors of AD, namely APOEe4 and MAPTA alleles, on resting-state functional connectivity in a cohort of 129 cognitively intact young adults (aged 17–22 years). We used Independent Component Analysis to identify networks of interest, and Gaussian Random Field Theory to compare connectivity between groups. Seed-based analysis was used to quantify inter-regional connectivity strength from the clusters that exhibited significant between-group differences. To investigate the relationship with cognition, we correlated the connectivity and the performance on the Stroop task.
Results
The analysis revealed a decrease in functional connectivity in the Default Mode Network (DMN) in both APOEe4 carriers and MAPTA carriers in comparison with non-carriers. APOEe4 carriers showed decreased connectivity in the right angular gyrus (size = 246, p-FDR = 0.0079), which was correlated with poorer performance on the Stroop task. MAPTA carriers showed decreased connectivity in the left middle temporal gyrus (size = 546, p-FDR = 0.0001). In addition, we found that only MAPTA carriers had a decreased connectivity between the DMN and multiple other brain regions.
Conclusions
Our findings indicate that APOEe4 and MAPTA alleles modulate brain functional connectivity in the brain regions within the DMN in cognitively intact young adults. APOEe4 carriers also showed a link between connectivity and cognition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s Disease (AD) affects an increasing number of people globally with no effective disease-modifying therapy currently available. Literature indicates the presence of brain changes related to AD decades before the onset of observable clinical symptoms [1]. Investigating early disease biomarkers is, therefore, crucial in prevention efforts, in understanding the nature of the disorder and for developing potential therapeutic interventions which might include risk factor modification.
Neuroimaging data has widened our understanding of the pathophysiology of AD and demonstrated the potential of developing neuroimaging-related biomarkers [2] including those based on network connectivity [3]. Resting-state networks have been observed to be already disrupted in the early stages of AD and deteriorate as the disease progresses [4]. Such changes are also present in healthy individuals with non-modifiable risk factors associated with AD in similar networks and brain areas across adulthood [5]. Notably, most studies report that connectivity changes already occur in middle-aged APOEe4 carriers. However, how early those AD-related changes occur prior to middle age is still not clear. Further robust evidence from other genetic risk factors in early life is also lacking.
The evidence from published literature focuses on the Default Mode Network (DMN) analyses due to its spatial overlap with amyloid distribution and tau pathology [6]. Brain areas of the DMN exhibit coherent activity when the individual’s focus of attention is not attributable to specific stimuli. The DMN activity has been linked with internal processes such as mental stimulations, introspection, or autobiographical retrieval and might be heterogenous across the network [6].
The DMN seems to be consistently disrupted in individuals who have genetic risk factors for AD; yet the directionality of those changes is inconclusive [5]. Similarly, the areas of the DMN display different functional connectivity in healthy individuals with AD-related amyloid [7] and tau pathology [8]. Other resting-state networks that show involvement in at-risk populations include the executive networks [9, 10] and the salience network [9,10,11]. Connectivity in resting-state networks also correlated with the performance on a set of cognitive tasks in individuals with AD-related pathology. For example, alterations in executive functions and inhibition control assessed as performance on the Stroop task in cognitively healthy individuals with amyloid pathology were linked with a decrease in DMN connectivity [12].
In this study, we have investigated the differences in resting-state functional connectivity between young carriers of genetic risk factors for AD and non-carriers using a candidate-gene approach (i.e., APOE and MAPT genes). These genes were selected as APOEe4 is consistently reported as a high-risk factor for AD [13], and MAPT is involved in tau production, which is aberrantly aggregated in AD and related tauopathies [14].
Additionally, the effect of connectivity and genotype on the Stroop task performance is investigated. Here, the Stroop task was chosen because it is widely used in both clinical and research settings as a reliable benchmark cognitive test in healthy young adults, for whom most clinical neuropsychological tools are unable to detect a change. It assesses selective attention, inhibition control, processing speed, cognitive load, and brain automaticity. Furthermore, previous evidence suggests that the Stroop effect is a robust predictor of neurodegeneration [15].
Methods
Study sample
A total of 392 young and cognitively healthy self-declared Chinese Han college students were recruited as an extension of the PREVENT-Dementia study [16] from Southwest University in Chongqing, China. Research ethics was provided by Southwest University’s local ethics committee. All participants provided informed written consent. Self-declaring as Han Chinese was part of the inclusion/exclusion criteria of the recruitment.
All participants provided a saliva sample for DNA genotyping. The genotypes for APOE and MAPT (rs242557) were determined by the Mass Array system (Agena iPLEX assay, San Diego, United States). For the purpose of this study, the study population was dichotomised based on (1) the presence of at least one copy of the APOEe4 allele (“APOEe4 carriers”); (2) the presence of the MAPT A allele, so that MAPT A and MAPT AG were grouped into one group (“MAPTA carriers”); (3) the presence of the MAPT G allele, so that MAPT G and MAPT AG were grouped into one group (“MAPTG carriers”). Based on the literature [17], we predicted that APOEe4 carriers and MAPTA carriers would have an increased risk for dementia in late life.
A subset of 155 participants underwent neuroimaging. Anatomical images from participants with poor segmentation quality and functional MRI images with poor acquisition quality were excluded resulting in a final dataset of 129 participants for the subsequent analysis. Demographic information is summarised in Table 1. There were no significant differences in gender, age, and education between the groups.
Neuroimaging acquisition
MRI imaging data were acquired on a 3 T Siemens whole-body scanner at Southwest University. Participants underwent brain scans at rest while instructed to keep their eyes closed and not to think about anything specific. Resting-state echo planar images were obtained (35 slices; slice thickness: 3 mm, TR/TE: 2000 ms/30 ms, flip angle: 80°, FOV: 192 × 192 mm2, voxel size: 3 × 3 × 3 mm3, slice acquisition: interleaved, total 330 measurements). Additionally, T1-weighted anatomical images were obtained using the Magnetisation Prepared Rapid Gradient Echo (MPRAGE) protocol (160 slices, slice thickness: 1 mm, TR/TE: 2300 ms/2.98 ms, flip angle: 9°, FOV: 240 × 256 mm2, voxel size: 1 × 1 × 1 mm3).
Neuroimaging data pre-processing
Neuroimaging pre-processing and functional connectivity analysis were performed using the CONN software (https://www.nitrc.org/projects/conn, RRID:SCR_009550) and in-house MatLab scripts (MathWorks, Natrick, MA). The pre-processing sequence in the CONN included motion estimation and correction, correction for inter-slice differences in acquisition time, outlier detection, and segmentation. The anatomical and functional images were coregistered and normalised to a standard brain space (MNI152) and smoothed (8 mm FWHM). Denoising of functional data consisted of white matter and cerebrospinal fluid regression, followed by despiking and band-pass filtering (0.008–0.9 Hz).
Functional images were analysed to identify spatially independent and temporally coherent networks using the Independent Component Analysis (ICA) as described by Calhoun and colleagues [18]. First, a spatial–temporal Principal Component Analysis was performed to reduce fMRI data to a lower dimensionality, which allowed a reduced complexity while persevering most of the information content. A FastICA algorithm [19] with Tahn distribution was used for the estimation of independent spatial components and a GICA1 backprojection [18] was used for individual subject-level spatial map estimation. The number of independent components was set to 17 based on the MDL (i.i.d. sampling) criteria. The optimal number of components was estimated using the Infomax algorithm [20] implemented in the group ICA for fMRI toolbox (GIFT software v4.0b, http://icatb.sourceforge.net). Infomax uses a fixed non-linearity for a super-Gaussian distribution by maximising information transfer between an input and an output of networks. Each component was represented by a spatial map and temporal profile. The resulting maps were used to compute the individual subject components (i.e., back reconstruction of components).
The resting-state networks were identified through a spatial correlation across voxels with the predefined connectivity network templates in the CONN, while the components with the highest spatial correlations were selected. This resulted in 7 resting-state networks: the DMN, sensorimotor, visual, salience, dorsoattentional, frontoparietal (i.e., executive network), and language networks (Fig. 1). The visualisation of the spatial correlations with the templates is provided in the Supplementary Material. Notably, both the salience network and the frontoparietal network showed a lower correlation to the network templates than the rest of the networks.
Between-group analyses
The selected networks were analysed in second-level within-network analyses with groups as factors (APOEe4 carriers vs non-carriers, MAPTA carriers vs non-carriers, and MAPTG carriers vs non-carriers) and age, gender, years of education and reported family history of dementia as covariates. Family history of dementia was determined by a self-reported diagnosis of dementia for the participants’ grandparents. We compared resting-state connectivity in all identified resting-state networks between groups by using parametric Gaussian Random Field Theory [21] with cluster FDR-corrected threshold < 0.05 implemented in CONN.
In the next step, seed-based analyses were conducted using the clusters that showed significant between-group differences as seeds. Connectivity values (i.e., calculated as the mean values of time-series) from respective significant clusters were compared with the connectivity values of the rest of the brain, quantifying inter-regional connectivity strength.
To explore the effect of connectivity and genotype on the Stroop task performance, the Stroop performance was calculated as both the accuracy in the colour-incongruent condition, and as the difference between reaction times in colour-incongruent and colour-congruent conditions. Behaviourally, comparisons of Stroop performance between groups were analysed using students’ t-tests. To explore whether Stroop performance varied among individuals as a function of APOE/MAPT status interacting with connectivity in each cluster, several linear models were built. In one set of models, Stroop performance was either explained by categorical group status, functional connectivity values in respective clusters, or their interactions. In another set of models, age, gender, education, and family history were additionally used to explain Stroop performance.
Results
Within-network and seed-based functional connectivity
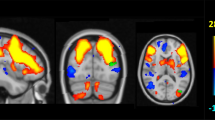
The DMN was the only network that showed significant differences between groups. There was a decreased connectivity in APOEe4 carriers compared with non-carriers in a cluster that comprised of the right angular gyrus and right superior occipital cortex (Cluster 1; size = 246 voxels, p-FWE = 0.0081, p-FDR = 0.0079; Fig. 2A). Similarly, MAPTA carriers showed decreased connectivity in the DMN in comparison with non-carriers in the left middle temporal gyrus cluster (Cluster 2; size = 546 voxels, p-FWE < 0.0001, p-FDR = 0.0001; Fig. 2B). The visual rendering of these clusters is presented in Fig. 3. No statistically significant between-group differences were observed between MAPTG -carriers. We additionally controlled for the effect of APOE in the MAPT analysis and vice versa. The resulting group differences were not changed.
The spatial maps of clusters that exhibited significant differences in connectivity between carriers and non-carriers. A Cluster 1 comprising of the right angular gyrus and superior occipital cortex that showed significant differences between APOEe4 carriers and non-carriers. B Cluster 2 comprising of the left middle temporal gyrus that showed significant differences between MAPTA carriers and non-carriers
The clusters that exhibited significant between-group differences were selected for further seed-based analyses to explore the global connectivity of the rest of the brain with these DMN regions. After assessing for between-group differences, no significant differences in connectivity of Cluster 1 to other brain areas were found between APOEe4 carriers and non-carriers. However, Cluster 2 exhibited an additional decrease in functional connectivity into multiple areas across the brain in MAPTA carriers. Namely, additionally decreased connectivity was observed in the right posterior and anterior middle temporal gyrus (size = 502 voxels, p-FWE = 0.0001, p-FDR = 0.0003), left posterior and anterior middle temporal gyrus (size = 314 voxels, p-FWE = 0.0029, p-FDR = 0.0019), right cerebellum (size = 457 voxels, p-FWE = 0.0002, p-FDR = 0.0003), left cerebellum (size = 222 voxels, p-FWE = 0.0169, p-FDR = 0.0077), precuneus and posterior cingulate cortex (size = 251 voxels, p-FWE = 0.0095, p-FDR = 0.0052), and in left frontal pole and frontal medial cortex (size = 393 voxels, p-FWE = 0.0007, p-FDR = 0.0006). The results are summarised in Table 2, and the visual rendering of all regions that exhibited significant between-group differences in global connectivity with the DMN is shown in Fig. 4.
Stroop performance affected by functional connectivity and genotypes
Eleven participants were excluded from the analysis due to not completing the cognitive tasks. Two of those participants were APOEe4 carriers and eight were MAPTA carriers.
The between-group analysis of the performance on the Stroop task did not reveal any significant differences in accuracy in the colour-incongruent condition between the APOEe4 carriers and non-carriers (t(116) = 0.44, p > 0.05), MAPTA carriers and non-carriers (t(116) = − 0.03, p > 0.05), or MAPTG carriers and non-carriers (t(116) = − 0.01, p > 0.05). Similarly, the analysis did not reveal any significant differences when Stroop performance was calculated as the difference between reaction times in colour-incongruent and colour-congruent conditions (APOEe4 carriers and non-carriers (t(116) = − 0.62, p > 0.05), MAPTA carriers and non-carriers (t(116) = 0.56, p > 0.05), and MAPTG carriers and non-carriers (t(116) = − 0.16, p > 0.05)).
We were further interested in exploring whether Stroop performance (i.e., accuracy in the colour-incongruent condition) varied among individuals as a function of APOE/MAPT status interacting with connectivity in each cluster. Several linear models were built in which Stroop performance was either explained by categorical group status, functional connectivity values in respective clusters, or their interactions.
After fitting the models for APOE, we observed that the model that contained the interaction between APOE status and connectivity in Cluster 1 explained the Stroop task performance significantly better than the model without the interaction (RSS = 0.221, sum of sq = 0.011, F = 5.787, p = 0.018). Specifically, we observed significant effects of APOE status (t = − 2.181, p = 0.031), connectivity in Cluster 1 (t = − 2.232, p = 0.028), and their interaction on Stroop performance (t = 2.417, p = 0.017). Neither age, gender, education, nor family history improved the performance of the model (p > 0.05). The interaction suggested that with any additional decrease in connectivity, there is a decrease in Stroop performance for APOEe4 carriers (coefficient estimate for the interaction = 0.024, p = 0.015).
After fitting MAPT models, the performance of the models did not differ significantly (p > 0.5). Adding age, gender, education, or family history did not improve the performance of the model (p > 0.05). These results were in line with the results observed by using Spearman’s correlation of Stroop performance and DMN connectivity (Fig. 5).
Spearman’s correlations between functional connectivity in respective clusters and Stroop performance (i.e., the accuracy in the colour-incongruent condition) between groups with fitted linear regression lines. APOEe4 carriers showed a positive relationship between connectivity in Cluster 1 and Stroop performance (R = 0.42, p = 0.037), while no other relationship was observed in APOEe4 non-carriers (R = − 0.16, p > 0.05), MAPTA carriers (R = 0.08, p > 0.05), and MAPTA non-carriers (R = 0.2, p > 0.05)
We further repeated the analyses by calculating Stroop performance as the difference in reaction times between the conditions (i.e., colour-incongruent and colour-congruent). The Stroop performance did not vary as a function of the APOE or MAPT, connectivity, or their interaction (p > 0.05). Neither age, gender, education, nor family history improved the performance of the model (p > 0.05).
Discussion
The present study used ICA and Gaussian Random Theory to study functional connectivity differences in carriers of genetic risk factors for AD. Our results indicated decreased functional connectivity in the DMN in the angular gyrus and lateral occipital areas in APOEe4 carriers in comparison with non-carriers. Comparing the inter-regional connectivity strength of this area with that of the rest of the brain did not reveal any additional disruptions of global brain connectivity. Compared to MAPTA non-carriers, MAPTA carriers displayed decreased functional connectivity in the DMN in the left middle temporal gyrus with additional disruptions in global brain connectivity in the areas that are often linked with AD-related pathology (e.g., precuneus, posterior cingulate cortex, and right middle temporal gyrus). Moreover, we observed a relationship between connectivity and cognition in APOEe4 carriers which was not observed in MAPTA carriers. This could suggest different underlying mechanisms of the genetics-related vulnerability of the brain.
In previous research, young APOEe4 carriers showed consistent functional connectivity disturbances in the DMN; yet, the directionality of the findings is inconclusive in the literature. For instance, studies with a methodology similar to that of the current study showed contradicting increased connectivity in young APOEe4 carriers in multiple DMN areas [22,23,24]. One of the factors that could add to the observed discrepancy between our findings and available literature could be the younger age of our participants (mean age: 19.6 years) than that in studies with similar methodology (21.4 years, 28.6 years, 23.8 years, respectively). Although not notably large, this difference might be crucial due to the evidence of large-scale network stabilisation that continues until early adulthood [25] and may differ between sexes [26]. In our study, we did not run additional analyses based on gender as this would result in insufficient power in some groups, especially in APOEe4 carriers. However, when using gender as one of the covariates in the models, the performance did not change significantly.
A systematic review of MRI-related brain changes also revealed that there is generally only limited evidence covering the age range between 18 and 21 years [27], which makes comparisons with other functional neuroimaging changes in the DMN regions additionally challenging. For instance, there are only two recent functional neuroimaging studies with a mean age of APOEe4 adult carriers below 20 years. They reported increased functional connectivity in the posterior regions of the DMN [28] and in the medial temporal regions [29] while using the same sample and a discrimination task.
There are two notable differences between our sample and those of the aforementioned studies. First, the total sample size of our study (n = 129) was larger than that of other similar studies. To our knowledge, there are only two resting-state studies investigating the effect of genetic risk factors on functional connectivity with a similar [30] or a larger total sample size [31]. The mean ages of APOEe4 carriers in these studies are 28.8 and 22.8 years respectively. Using different methodologies, both studies yielded mixed results. While there was no difference in the functional connectivity between young APOEe4 carriers and non-carriers, [30], there was both a decrease and increase in connectivity between various brain areas in APOEe4-KIBRA carriers [31].
Another difference is linked to the ethnicity of the participants. Previous research suggests that APOEe4 may modulate DMN connectivity differently in different ethnicities [32]. While the ethnic background of the participants in studies with contradictory findings is unclear, the majority recruited their participants in the UK [22, 28, 29]. It can be assumed that their ethnicity differed from that of the Chinese Han. Nevertheless, there were only two other resting-state functional connectivity studies with ethnic Chinese Han samples [30, 31]. The findings of the two studies are discussed above.
Interestingly, no previous studies in at-risk young populations indicated considerable connectivity differences in the angular gyri. However, decreased functional connectivity in these areas was observed in converters with Mild Cognitive Impairment [33]. The structures of the angular gyri have been also previously linked with energy metabolism changes that could predict a decline in global cognitive function [34]. We also observed a moderate relationship between the performance on the Stroop task and a decline in functional connectivity in the right angular gyrus cluster when calculating the Stroop performance as the accuracy in the colour-incongruent condition. This relationship indicated that with decreasing functional connectivity, the accuracy in the colour-incongruent condition in the Stroop task additionally worsened. One potential explanation for this relationship is the binding role of the angular gyrus between different domains and processing streams (e.g., visuospatial, somatosensory, auditory) that are involved in multiple cognitive functions [35]. Reduced connectivity in these areas may compromise this binding function, resulting in reduced accuracy in the Stroop task.
Notably, when calculating Stroop performance as the reaction time difference between the conditions, we did not observe this relationship. Such discrepancy has been observed in multiple different paradigms such as tactile decision-making [36, 37] and could be explained by the employment of different cognitive and neural mechanisms.
Reduced functional connectivity in individuals with MAPT genetic risk factors in the medial temporal areas is mostly observed in studies targeting behavioural variants of frontotemporal dementia [38]. Little is known about the relationship between functional connectivity and the MAPT gene in patients with AD. Some evidence from animal models of early-stage AD suggests functional connectivity disturbances in the medial temporal regions [39]. AppNL-F/MAPT double knock-in mice displayed disturbances in brain network organisation within the medial temporal regions and an additional global reduction of connectivity to other brain regions, which is in line with our results. The limited empirical evidence of the relationship between MAPT, AD, and connectivity disturbances should be taken into consideration when interpreting the present results. This is an important issue for future research that should consider a wider range of genetic and environmental risk factors of AD beyond the APOEe4 allele to answer further questions about the underlying mechanisms of related connectivity changes. GWAS and polygenic risk score studies could also be important additions to more efforts in personalised medicine.
Intriguingly, there was no significant difference in functional connectivity between MAPTG carriers and non-carriers. This suggests that grouping the AG allele with other genotypes has a significant impact on the results. We recommend considering this in future studies, similar to accounting for the impact of ethnicity on genetic risk.
The limitations of the present study relate to technical aspects of the analytical toolbox of choice (CONN) which does not provide the network stability assessment (e.g., by using the ICASSO algorithm). Additionally, it does not provide the correlation values of the spatial correlation of independent components with the network templates beyond the visualisation. This makes the stability and reliability assessment of the identified networks difficult and should be taken into account. For instance, the salience network and the frontoparietal network show lower correlations with the templates in comparison to other networks in the study which should be carefully considered when interpreting the results.
Conclusion
Our findings offer an important insight into the functional connectivity patterns across the lifespan, specifically in young adulthood, while adding to the limited evidence of the role of genetic risk factors for AD in functional brain changes. Whilst MAPTA carriers demonstrated more global disturbances in functional connectivity, APOEe4 carriers showed a link between functional connectivity and cognition. Hence, our results suggest that while both APOEe4 and MAPTA alleles modulate brain functional connectivity in young adults, they may do so via different underlying mechanisms.
References
Jack CR et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12(2):207–216. https://doi.org/10.1016/S1474-4422(12)70291-0
Márquez F, Yassa MA (2019) Neuroimaging biomarkers for Alzheimer’s disease. 5:1–14
Gomez-Ramirez J, Wu J (2014) Network-based biomarkers in Alzheimer’s disease: review and future directions. Front Aging Neurosci 6:1–9. https://doi.org/10.3389/fnagi.2014.00012
Yildirim E, Büyükişcan ES (2019) Default mode network connectivity in Alzheimer’s disease. Turk Psikiyatr Derg 30(4):279–286. https://doi.org/10.5080/u23526
Kucikova L et al (2021) Resting-state brain connectivity in healthy young and middle-aged adults at risk of progressive Alzheimer’s disease. Neurosci Biobehav Rev 129(May):142–153. https://doi.org/10.1016/j.neubiorev.2021.07.024
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. https://doi.org/10.1196/annals.1440.011
Sheline YI et al (2010) APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci 30(50):17035–17040. https://doi.org/10.1523/JNEUROSCI.3987-10.2010
Quevenco FC et al (2020) Functional brain network connectivity patterns associated with normal cognition at old-age, local β-amyloid, tau, and APOE4. Front Aging Neurosci 12(March):1–11. https://doi.org/10.3389/fnagi.2020.00046
Goveas JS et al (2013) Functional network endophenotypes unravel the effects of apolipoprotein E epsilon 4 in middle-aged adults. PLoS ONE. https://doi.org/10.1371/journal.pone.0055902
Wu X et al (2016) A triple network connectivity study of large-scale brain systems in cognitively normal APOE4 carriers. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2016.00231
Lee SE et al (2019) Thalamo-cortical network hyperconnectivity in preclinical progranulin mutation carriers. NeuroImage Clin 22:101751. https://doi.org/10.1016/j.nicl.2019.101751
Duchek JM et al (2013) Relationship between stroop performance and resting state functional connectivity in cognitively normal older adults. Neuropsychology 27(5):516–528. https://doi.org/10.1037/a0033402
Bertram L, Tanzi RE (2008) Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 9(10):768–778. https://doi.org/10.1038/nrn2494
Strang KH, Golde TE, Giasson BI (2019) MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Investig 99(7):912–928. https://doi.org/10.1038/s41374-019-0197-x
Bélanger S, Belleville S, Gauthier S (2010) Inhibition impairments in Alzheimer’s disease, mild cognitive impairment and healthy aging: effect of congruency proportion in a Stroop task. Neuropsychologia 48(2):581–590. https://doi.org/10.1016/j.neuropsychologia.2009.10.021
Ritchie CW, Wells K, Ritchie K (2013) The PREVENT research programme-A novel research programme to identify and manage midlife risk for dementia: the conceptual framework. Int Rev Psychiatry 25(6):748–754. https://doi.org/10.3109/09540261.2013.869195
Zhou F, Wang D (2017) The associations between the MAPT polymorphisms and Alzheimer’s disease risk: a meta-analysis. Oncotarget 8(26):43506–43520. https://doi.org/10.18632/oncotarget.16490
Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2002) Erratum: A method for making group inferences from functional mri data using independent component analysis (Human Brain Mapping (2001) 14 (140–151)). Hum Brain Mapp 16(2):131. https://doi.org/10.1002/hbm.10044
Hyvärinen A (1999) Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Networks 10(3):626–634. https://doi.org/10.1109/72.761722
Bell AJ, Sejnowski TJ (1995) An information-maximization approach to blind separation and blind deconvolution. Neural Comput 7(6):1129–1159. https://doi.org/10.1162/neco.1995.7.6.1129
Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996) A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4(1):58–73. https://doi.org/10.1002/(SICI)1097-0193(1996)4:1%3c58::AID-HBM4%3e3.0.CO;2-O
Dowell NG, Evans SL, Tofts PS, King SL, Tabet N, Rusted JM (2016) Structural and resting-state MRI detects regional brain differences in young and mid-age healthy APOE-e4 carriers compared with non-APOE-e4 carriers. NMR Biomed 29(5):614–624. https://doi.org/10.1002/nbm.3502
Filippini N et al. (2009) Distinct patterns of brain activity in young carriers of the APOE-4 allele. 106(17):7209–7214
Su YY et al (2015) APOE polymorphism affects brain default mode network in healthy young adults: a STROBE article. Medicine. https://doi.org/10.1097/MD.0000000000001734
Edde M, Leroux G, Altena E, Chanraud S (2021) Functional brain connectivity changes across the human life span: from fetal development to old age. J Neurosci Res 99(1):236–262. https://doi.org/10.1002/jnr.24669
Ernst M et al (2019) Pubertal maturation and sex effects on the default-mode network connectivity implicated in mood dysregulation. Transl Psychiatry. https://doi.org/10.1038/s41398-019-0433-6
Hedman AM, van Haren NEM, Schnack HG, Kahn RS, Hulshoff Pol HE (2012) Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 33(8):1987–2002. https://doi.org/10.1002/hbm.21334
Hodgetts CJ et al (2019) Increased posterior default mode network activity and structural connectivity in young adult APOE-ε4 carriers: a multimodal imaging investigation. Neurobiol Aging 73:82–91. https://doi.org/10.1016/j.neurobiolaging.2018.08.026
Shine JM, Muller AJ, O’Callaghan C, Hornberger M, Halliday GM, Lewis SJG (2015) Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson’s disease: a task-based fMRI study. Parkinsons Dis. https://doi.org/10.1038/npjparkd.2015.3
Yang AC et al (2014) The APOE e{open}4 allele affects complexity and functional connectivity of resting brain activity in healthy adults. Hum Brain Mapp 35(7):3238–3248. https://doi.org/10.1002/hbm.22398
Zhang N, Liu H, Qin W, Liu B, Jiang T, Yu C (2017) APOE and KIBRA interactions on brain functional connectivity in healthy young adults. September 2016, pp 4797–4805, doi: https://doi.org/10.1093/cercor/bhw276.
Turney IC et al (2020) APOE ε4 and resting-state functional connectivity in racially/ethnically diverse older adults. Alzheimer’s Dement Diagn Assess Dis Monit 12(1):1–8. https://doi.org/10.1002/dad2.12094
Li Y et al (2016) Abnormal resting-state functional connectivity strength in mild cognitive impairment and its conversion to Alzheimer’s disease. Neural Plast. https://doi.org/10.1155/2016/4680972
Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M (2006) Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol 59(4):673–681. https://doi.org/10.1002/ana.20799
Seghier ML (2013) The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19(1):43–61. https://doi.org/10.1177/1073858412440596
van Ede F, de Lange FP, Maris E (2012) Attentional cues affect accuracy and reaction time via different cognitive and neural processes. J Neurosci 32(30):10408–10412. https://doi.org/10.1523/JNEUROSCI.1337-12.2012
Mulder MJ, Keuken MC, van Maanen L, Boekel W, Forstmann BU, Wagenmakers EJ (2013) The speed and accuracy of perceptual decisions in a random-tone pitch task. Attention Percep Psychophys 75(5):1048–1058. https://doi.org/10.3758/s13414-013-0447-8
Whitwell JL et al (2011) Altered functional connectivity in asymptomatic MAPT subjects a comparison to bvFTD. Neurology 77(9):866–874. https://doi.org/10.1212/WNL.0b013e31822c61f2
Borcuk C et al (2022) Early memory deficits and extensive brain network disorganization in the App/MAPT double knock-in mouse model of familial Alzheimer’s disease. Aging Brain 2:100042. https://doi.org/10.1016/j.nbas.2022.100042
Acknowledgements
LK conducted the analyses. LK and LS drafted the manuscript. JZ collected the data. CR, SG, LS and JZ set up the extension of the PREVENT-Dementia study. CMN pre-processed neuroimaging data. WH translated the databases of cognitive data. GMT and WH provided feedback on the analysis. CMN, GMT, WH, CR, SG, JOB provided feedback on the manuscript. LS overlooked the study. The analysis was conducted by using High Performance Computing services provided by the University of Sheffield.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
LK is a recipient of the University of Sheffield Flagship Scholarship. LS’s participation is funded by Alzheimer's Research UK Senior Research Fellowship (ARUK-SRF2017B-1), NIHR Sheffield Biomedical Research Centre. JZ, CMN, GMT, and WH have no financial conflicts of interests to disclose. JOB has acted as a consultant for TauRx, Novo Nordisk, Biogen, Roche, Lilly and GE Healthcare and received grant or academic support from Avid/ Lilly, Merck and Alliance Medical. CR is the founder of Scottish Brain Sciences, and acted as a consultant for Biogen, Eisai, MSD, Actinogen, Roche, and Eli Lilly, and received payment or honoraria from Roche and Eisai in the past. SG acted as a consultant for Scottish Brain Sciences.
Ethical approval
The study was approved by the Human Ethics Committee for Psychological Research at Southwest University (H17036). All participants provided informed consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kucikova, L., Zeng, J., Muñoz-Neira, C. et al. Genetic risk factors of Alzheimer’s Disease disrupt resting-state functional connectivity in cognitively intact young individuals. J Neurol 270, 4949–4958 (2023). https://doi.org/10.1007/s00415-023-11809-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11809-9