Abstract
Introduction
In malignant cerebral infarction decompressive hemicraniectomy has demonstrated beneficial effects, but the optimum size of hemicraniectomy is still a matter of debate. Some surgeons prefer a large-sized hemicraniectomy with a diameter of more than 14 cm (HC > 14). We investigated whether this approach is associated with reduced mortality and an improved long-term functional outcome compared to a standard hemicraniectomy with a diameter of less than 14 cm (HC ≤ 14).
Methods
Patients from the DESTINY (DEcompressive Surgery for the Treatment of malignant INfarction of the middle cerebral arterY) registry who received hemicraniectomy were dichotomized according to the hemicraniectomy diameter (HC ≤ 14 cm vs. HC > 14 cm). The primary outcome was modified Rankin scale (mRS) score ≤ 4 after 12 months. Secondary outcomes were in-hospital mortality, mRS ≤ 3 and mortality after 12 months, and the rate of hemicraniectomy-related complications. The diameter of the hemicraniectomy was examined as an independent predictor of functional outcome in multivariable analyses.
Results
Among 130 patients (32.3% female, mean (SD) age 55 (11) years), the mean hemicraniectomy diameter was 13.6 cm. 42 patients (32.3%) had HC > 14. There were no significant differences in the primary outcome and mortality by size of hemicraniectomy. Rate of complications did not differ (HC ≤ 14 27.6% vs. HC > 14 36.6%, p = 0.302). Age and infarct volume but not hemicraniectomy diameter were associated with outcome in multivariable analyses.
Conclusion
In this post-hoc analysis, large hemicraniectomy was not associated with an improved outcome or lower mortality in unselected patients with malignant middle cerebral artery infarction. Randomized trials should further examine whether individual patients could benefit from a large-sized hemicraniectomy.
Clinical trial registration information
German Clinical Trials Register (URL: https://www.drks.de; Unique Identifier: DRKS00000624).
Similar content being viewed by others
Introduction
In malignant cerebral infarction space-occupying brain edema is associated with a mortality of up to 80% despite intensive care treatment [4]. Early hemicraniectomy within 48 h of symptom onset has demonstrated improved functional outcome and to reduced mortality in randomized controlled trials (RCTs) [18, 22]. Despite the beneficial effects of this intervention, about 20% of patients still die from herniation in the acute phase after hemicraniectomy, which theoretically might be avoided by a larger hemicraniectomy resulting in additional relief for the swollen brain tissue [8, 22].
The volume gained by hemicraniectomy directly correlates with the diameter of the bone flap. Current recommendations are based on in vitro models which calculated a minimum diameter of 12 cm to create an additional volume of at least 80 ml [28]. While hemicraniectomies with a diameter of less than 12 cm may bear the risk of transcalvarial herniation and hemicraniectomy-associated hemorrhages due to shear injury at the bone edges, larger bone flaps of more than 14 cm in diameter may be associated with more surgical complications such as bleedings, damage to bridging veins or sinus, postsurgical hydrocephalus, and the sinking skin flap syndrome [10, 26]. Several retrospective studies reported controversial data about the association of larger bone flaps with improved functional outcome or mortality, but were all limited by retrospective single-center designs and small sample sizes [1, 6, 11, 14, 20]. Nevertheless, current national and international guidelines for management of large hemispheric infarction recommend a standard diameter of at least 12 cm, and suggest a larger diameters of 14–16 cm with a moderate quality of evidence [21, 24]. Thus, the optimal diameter of hemicraniectomy remains to be defined. The present study used prospectively collected data from a multicenter registry to evaluate long-term functional outcome and mortality as well as safety of an enlarged hemicraniectomy with a diameter of more than 14 cm (HC > 14) compared to a diameter of less than 14 cm (HC ≤ 14).
Methods
Between January 2010 and July 2016 patients with large ischemic infarction were prospectively included into the DEcompressive Surgery for the Treatment of malignant INfarction of the middle cerebral arterY—Registry (DESTINY-R) study in 30 neurological and neurosurgical departments in Germany and Austria.
A detailed description of the study design has been published previously [15]. The trial was registered in the German Registry for Clinical Studies (DRKS00000624). The ethics committee of the Charité – Universitätsmedizin Berlin, Campus Benjamin Franklin (EA4/108/09) and the local ethics committees of all participating centers approved this registry. Written informed consent was obtained from all patients or legal representatives.
For this analysis patients who fulfilled the following inclusion criteria were selected from the complete study cohort: (1) ischemic infarction of at least 50% of the middle cerebral artery territory confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) with corresponding clinical signs of a severe hemispheric syndrome, with or without additional infarction of the ipsilateral anterior or posterior cerebral artery territory, (2) hemicraniectomy performed according to the decision of the treating physician independent of study participation, (3) available postoperative imaging data measuring hemicraniectomy diameter, and (4) completed follow-up assessment of functional outcome conducted after 12 months. Patients with concomitant acute contralateral and/or infratentorial infarction or additional acute traumatic brain injury were excluded. The timing of hemicraniectomy and choice of the surgical technique (including the diameter of the bone flap, type of duraplasty) were left to the discretion of the treating neurosurgeon with a reference to the current guidelines [21].
After study inclusion, sociodemographic factors, clinical parameters (stroke severity on admission, level of consciousness on admission and before neurosurgery), pre-morbid functional status, vascular risk factors as well as surgical and medical treatment data were documented in a case report form [3]. Additionally, relevant parameters of hemicraniectomy as well as complications of neurosurgery and during intensive care treatment were documented. Follow-up assessment was performed via a structured telephone interview 12 months after stroke onset.
Clinical outcome analysis
The primary outcome was defined as the functional status according to the modified Rankin Scale score (mRS) after 12 months. Clinical outcome was dichotomized as mRS 0–4 versus 5 and 6 according to the definition of the pooled analysis of the three European hemicraniectomy RCTs [22]. Secondary outcome measures were (1) in-hospital mortality, (2) mortality within 12 months after stroke onset, (3) complications regarding intensive care treatment or neurosurgery and (4) functional outcome dichotomized as mRS 0–3 versus 4–6 [13, 16]. In addition, a subgroup analysis was performed in younger patients ≤ 60 years.
Neuroimaging analysis
Neuroradiological parameters were processed centrally and blinded to functional outcome on the basis of CT or MRI scans on admission and after neurosurgery using TIFT (Tension Imaging and Fiber Tracking) software [12, 17, 19]. After imputation of imaging data all scans were transformed into a three-dimensional grid with a voxel size of 0.5 mm by nearest-neighbour-transformation and the brain was aligned along a horizontal line between the anterior and posterior commissure. For the volumetric measurement of infarct volume at inclusion semi-automatically three-dimensional delineation of hypodense tissue on CT and hyperintensity on diffusion-weighted MRI was used to identify lesion-related voxels. Subsequently, opening and closing procedures were performed to delineate a homogeneous infarct region and to exclude false positive voxels. In order to ensure the comparability of the calculated volumes, infarct volume was standardized for total intracranial volume [27]. In contrast, hemicraniectomy diameter was not standardized, because the neurosurgeons used absolute values as a guide during the operation.
To simplify the measurement of the hemicraniectomy diameter on postoperative imaging data, the bone flap was considered as an ellipse oriented in sagittal axis (see Fig. 1): First, the image showing the largest diameter of the bone flap in the axial plane was selected and aligned along the sagittal axis (larger axis = major). Second, the same procedure was performed in the coronal plane to define the smaller axis (minor). Subsequently, the ellipse was rotated in the sagittal plane to obtain the minor and major axes directly from coronal and axial coordinates (Fig. 1). The diameter of the hemicraniectomy was defined as the length (L) of the ellipse´s major axis, while height (H) was defined as the ellipse´s smaller axis (minor). Surface area (A) of the hemicraniectomy was calculated using the two perpendicular parameters and the following formula: A = L/2 × H/2 × π.
Non-contrast computed tomography CT scan in representative slices after alignment (rotation angles ϕ1, ϕ2, ϕ3) of the trephination hole: A “sagittal” slice aligned with the trephination hole as an ellipse for calculation of the surface area (light blue); B “coronal” slice with the height of the minor axis (H, green); C “axial” slice with the major axis defining the “diameter” of the hemicraniectomy (L, red)
All patients were dichotomized in two groups based on the diameter of the bone flap according to the larger major axis defining diameter ≤ 14 cm as “standard hemicraniectomy” (HC ≤ 14) and > 14 cm as “large hemicraniectomy” (HC > 14) [11, 14, 21]. Additionally, amount of midline shift and involvement of anterior/posterior cerebral artery territory, and uncal herniation at study inclusion were assessed on pre-operative neuroimaging.
Statistical analysis
Statistics were performed using the SPSS 26 software package (SPSS Inc., Chicago, Illinois, USA). Descriptive analyses were calculated for all variables. Student´s t test, Mann–Whitney U test, or χ2 test were used as appropriate to identify differences between the two treatment-groups (HC ≤ 14 vs. HC > 14). To prevent confounding by indication, i.e., performing larger diameter of hemicraniectomy for larger infarctions, linear regression analysis between infarct volume at study inclusion and diameter of hemicraniectomy was performed. Using receiver-operating characteristic (ROC) analysis, threshold levels of hemicraniectomy diameter were calculated to evaluate the cut-off suggested by current guidelines of 14 cm. Diameter of bone flap and parameters of neurosurgery and conservative therapy were entered in a binary logistic regression model to evaluate their impact on functional outcome adjusted for age and infarct volume on admission. Results were expressed as odds ratios with 95%-Confidence Interval (OR, 95%-CI). The level of significance was defined as p < 0.05.
Results
Among 168 patients with hemicraniectomy, functional outcome data after 12 months and postsurgical imaging were available from 130 patients who were included in our analysis. Of these patients, 42 (32.3%) were female, mean age (SD) was 54.5 (11.0) years, and 94 (72.3%) patients were younger than 60 years at study inclusion. One year after hemicraniectomy 27 (20.8%) patients had a mRS of 0–3 and 79 (60.8%) patients had a mRS of 0–4. 29 (22.3%) patients had died before the 12 months follow-up. Causes of death during hospital stay (N = 15, 11.5%) were transtentorial herniation (N = 9), cardiac arrest (N = 2), pneumonia (N = 1), pulmonary embolism (N = 1), and unknown (N = 2). Causes of death during the follow-up period after discharge were not assessed. Depending on the decision of the operating neurosurgeon 88 (67.7%) patients underwent standard hemicraniectomy (≤ 14 cm) and 42 (32.3%) patients were treated by large hemicraniectomy (> 14 cm). Large hemicraniectomy was performed at 10 out of 30 study centers and 30 out of 42 (72%) cases were performed at 3 study centers. No significant differences in baseline demographic or clinical characteristics were found between the two hemicraniectomy groups (Table 1). Timing of neurosurgery after symptom-onset did not vary between treatment groups [HC ≤ 14: 29 (21–54) hours vs. HC > 14: 31 (21–50) hours, p = 0.799]. Furthermore, there was no difference in the median time interval between imaging and hemicraniectomy (HC ≤ 14: 6 (3–12) hours vs. HC > 14: 4 (2–15) hours, p = 0.372). Volume of infarction before neurosurgery (HC ≤ 14: 244 ± 63 ml vs. HC > 14: 241 ± 52 ml, p = 0.909), left hemispheric infarction (HC ≤ 14: 43.2% vs. HC > 14: 45.0%, p = 0.982) and concomitant involvement of the anterior cerebral artery (HC ≤ 14: 17.2% vs. HC > 14: 14.3%, p = 0.670) or posterior cerebral artery (HC ≤ 14: 4.6% vs. HC > 14: 4.8%, p = 0.967) territory was similar in both groups. Furthermore, there were no differences in the amount of midline shift (HC ≤ 14: 2.9 ± 3.9 mm vs. HC > 14: 2.3 ± 3.1 mm, p = 0.494) or frequency of uncal herniation (HC ≤ 14: 7.1% vs. HC > 14: 10.0%, p = 0.585) before neurosurgery. Medical treatment of elevated intracranial pressure differed regarding osmotic therapy (HC ≤ 14: 44.8% vs. HC > 14: 28.6%, p = 0.077) and muscle relaxation (HC ≤ 14: 26.4% vs. HC > 14: 9.5%, p = 0.027). In the HC > 14 group the diameter of the craniotomy (HC ≤ 14: 12.8 cm vs. HC > 14: 15.8 cm, p < 0.001) and the area of the craniotomy (HC ≤ 14: 94.7 cm2 vs. HC > 14: 119.4cm2, p < 0.001) were larger than in the HC ≤ 14 group (Table 2).
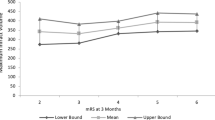
Table 2 shows the primary and secondary clinical outcome measures. There was no significant difference for the primary endpoint, i.e., mRS 0–4 after one year, between both treatment groups (HC ≤ 14: 60.2% vs. HC > 14: 61.9%, p = 0.855). Furthermore, neither in-hospital mortality nor mortality after 12 months follow-up period differed between both groups. In the HC > 14 group five of 42 patients (11.9%) achieved a mRS 0–3 at follow-up compared to 22 of 88 patients (25.0%) in the HC ≤ 14 group (p = 0.085). Similar results were also found in the subgroup of younger patients (≤ 60 years) and after exclusion of patients with hemicraniectomy after more than 48 h, respectively. Figure 2 illustrates the distribution of mRS scores after 12 months.
In linear regression analysis no association of infarct volume and diameter of bone flap was observed (F(1,118) = 0.216, p = 0.643), suggesting an absence of confounding by indication and the unselected use of large over standard hemicraniectomy. In addition, ROC analyses did not determine a certain hemicraniectomy diameter other than 14 cm that could predict outcome (mRS 0–4: AUC 0.53, 0.42–0.6, p = 0.612; mRS 0–3: AUC 0.46, 0.34–0.57, p = 0.482), in-hospital mortality (AUC 0.50, 0.35–0.65, p = 0.965), or mortality after 12 months (AUC 0.50, 0.38–0.61, p = 0.946) with a sensitivity or specificity higher than chance.
Neurosurgery-related parameters and complications regarding hemicraniectomy or intensive care are shown in Table 3. Type of duraplasty differed between treatment groups: if an enlarged hemicraniectomy was performed, the dura was more often rapidly closed and less often sewed. A similar number of patients experienced at least one complication related to neurosurgery (HC ≤ 14: 27.6% vs. HC > 14: 36.6%, p = 0.302), while the rate of patients with multiple complications was significantly higher after large hemicraniectomy (22.0% vs. 3.4%, p = 0.003). Although wound healing disorders [6 (14.6%) vs. 2(2.3%), p = 0.007] and subdural hematomas [5 (12.2%) vs. 2 (2.3%), p = 0.034] were more frequently observed after a large hemicraniectomy, these differences were small in terms of absolute numbers and observed in only few of the study centers. Furthermore, complications regarding hemicraniectomy equally often required another surgical intervention in both groups (HC ≤ 14: 5.7% vs. HC > 14: 9.8%, p = 0.408). No differences were found between treatment groups concerning duration of mechanical ventilation or frequency of intensive care-associated complications, e.g. pneumonia or deep vein thrombosis. Complications regarding cranioplasty, which was performed after a median of 116 (IQR 90–155) days, were more frequent in the HC > 14 group (70.6% vs. 44.1%, p = 0.012) and required more often another surgical intervention after HC > 14 (32.4% vs. 14.9%, p = 0.041).
In multivariable binary regression analyses with outcome as dependent variable, adjusted for age, volume of infarction, number of complications, frequency of wound healing disorders, frequency of subdural hematomas, differences in conservative therapy, and type of duraplasty performed, the diameter of hemicraniectomy was no significant predictor of outcome (Table 4). The additional inclusion of frequency and number of complications regarding cranioplasty had no impact on the results.
Discussion
Despite early prophylactic hemicraniectomy almost 20% of patients who suffer a malignant middle cerebral artery infarction still die. Most deaths occur early and are due to herniation which raises the question if some patients might benefit from a larger hemicraniectomy or if a larger hemicraniectomy in general could be beneficial [2, 4]. None of the hemicraniectomy RCTs provided data on the size of craniectomy or subgroup analyses concerning this issue. Only few data from observational studies are available comparing different sizes of craniectomy. Current guidelines recommend a diameter of the bone flap of at least 12 cm [24]. The results of the current post-hoc analysis from a prospective, multicenter registry could not demonstrate a benefit from a large hemicraniectomy regarding mortality or long-term functional outcome compared to a standard hemicraniectomy in unselected patients with malignant infarction. Furthermore, the data analyses could not determine a certain hemicraniectomy diameter that was meaningfully associated with any of the prespecified outcomes. However, these observations need to be interpreted with caution due to the low percentage of patients treated with large hemicraniectomy at only a few of the participating centers, and due to the post-hoc approach of this analysis.
Mortality in this study population was similar compared to patients treated with hemicraniectomy in the pooled analysis of RCTs in younger patients with malignant infarction [22]. Functional outcome on the other hand was worse in the present study. This finding is most probably due to the broader inclusion criteria of this multicenter registry, which compared to the RCTs better reflects the real-world demographics by including patients with higher age. This is further supported by findings of a recent patient-level meta-analysis [18].
Since infarct volume can predict outcome after decompressive hemicraniectomy [5], the association of a larger hemicraniectomy diameter and outcome in malignant infarction seems intuitive but so far has previously only been evaluated in six retrospective single center studies, which showed heterogenous results. In accordance with our results, three of these studies did not find an association of the size of craniectomy diameter with functional outcome, physical disability, or quality of life [2, 20, 25]. However, the number of patients included in our study was even larger. Two other studies reported potential benefits of larger hemicraniectomies. One study from 2011 found a significantly higher rate of patients with a mRS ≤ 3 in patients with a larger hemicraniectomy diameter. Here, a large landmark-based craniectomy with a mean diameter of 14.9 (± 0.9) cm was performed in 11 patients compared to a historical control of 13 patients following hemicraniectomy with a bone flap of a mean diameter of 12.6 (± 0.9) cm [1]. Another study from 2019 suggested a benefit in functional outcome (mRS ≤ 3) following a so called maximum hemicraniectomy with a diameter of more than 14 cm including resection of the temporalis muscle with its fascia and an expansive duraplasty in 14 younger patients with malignant infarction compared to outcome data from the literature [11]. However, considering not only the younger patients but the whole study cohort including 11 other elderly patients, only 36% of patients had a mRS ≤ 3 which is both comparable to the results of the latest meta-analysis of individual patient data (37%)[18] as well as to the results in the hemicraniectomy group of the current study (31%). In line with another study from 2016 with 97 patients following hemicraniectomy for malignant infarction, we did not observe a reduction in in-hospital mortality [14].
In RCTs, the rates of hemicraniectomy-related complications were comparatively lower (9% and 7%, respectively) [7, 23], than in observational studies, which show similar rates of complications as our study [9]. Although the complication rate was higher in patients treated with larger hemicraniectomy, we could not confirm an association of hemicraniectomy-associated complications and worse functional outcome with the larger diameter. Furthermore, studies reporting low complication rates and improved outcome following enlarged hemicraniectomy were conducted by experienced neurosurgeons in highly specialized centers and retrospectively evaluated [1, 11, 14].
In our cohort osmotherapy and muscle relaxation were applied more frequently in the standard hemicraniectomy group. Although both treatments had no impact on functional outcome in multivariable analyses, the increased use of osmotherapy and muscle relaxation in patients with smaller hemicraniectomies may be indicative of a poorer control of intracranial pressure compared to patients with larger bone flaps.
Despite the strengths of a prospective multicenter registry, our study is not without limitations: it is a non-randomized study and the decision of timing, diameter, and technique of the hemicraniectomy was left at the discretion of the treating neurosurgeon. Hemicraniectomy with duraplasty as a procedure was not standardized [10], neither were pre- and postoperative treatment algorithms, intensive care monitoring, extubation strategies, or the additional use of intracranial pressure lowering treatments including therapeutic hypothermia. Although this approach bears the risk of confounding, we did not observe differences in any of the clinical or radiological parameters used to determine the indication for hemicraniectomy such as midline shift, time since symptom onset, loss of consciousness, or stroke severity. Furthermore, because this is assumed to be standard neurosurgical practice, we did not evaluate an appropriate decompression of the temporal bone in the registry. However, uncal herniation after surgery was less frequent in both treatment groups, suggesting a comparable temporal bone decompression. Most importantly, hemicraniectomy diameter was also not associated with infarct volume which refutes the assumption that larger infarcts were treated by larger hemicraniectomies. In addition, through the inclusion of a wide range of university and community hospitals, treatment of malignant infarction was carried out by physicians with different clinical and surgical experience. Alternatively, the surface area could be calculated by automated segmentation tools; however, we decided to calculate the bone flap by manual approximation as an ellipse by manual approximation in order to obtain a visual control of the results. The surface area could then be calculated from the semi-major axis. The differences result from an easily determined parameter (diameter) that the treating neurosurgeon has at hand intraoperatively, whereas the determination of the surface area is a parameter which is difficult to reproduce intraoperatively. Finally, we did not assess the rate of application of advanced directives resulting in care limitations despite hemicraniectomy which may impact outcome [29].
In conclusion, our results do not provide evidence that large hemicraniectomy is beneficial regarding functional outcome or mortality as compared to standard hemicraniectomy in unselected patients with malignant middle cerebral artery infarction treated by hemicraniectomy. Although our results indicate that standard hemicraniectomy may be associated with less complications and is sufficient in most cases, selected patients may still benefit from a larger diameter hemicraniectomy to achieve the best possible outcome. This relevant question should be addressed in a randomized controlled trial.
Data availability
Original data are available and will be shared by request from a qualified investigator.
References
Chung J, Bang OY, Lim YC, Park SK, Shin YS (2011) Newly suggested surgical method of decompressive craniectomy for patients with middle cerebral artery infarction. Neurologist 17:11–15
Curry WT Jr, Sethi MK, Ogilvy CS, Carter BS (2005) Factors associated with outcome after hemicraniectomy for large middle cerebral artery territory infarction. Neurosurgery 56:681–692
Güresir E, Vatter H, Schuss P, Oszvald A, Raabe A, Seifert V, Beck J (2011) Rapid closure technique in decompressive craniectomy. J Neurosurg 114:954–960
Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R (1996) “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 53:309–315
Hecht N, Neugebauer H, Fiss I, Pinczolits A, Vajkoczy P, Juttler E, Woitzik J (2018) Infarct volume predicts outcome after decompressive hemicraniectomy for malignant hemispheric stroke. J Cereb Blood Flow Metab 38:1096–1103
Hinduja A, Samant R, Feng D, Hannawi Y (2018) Herniation despite decompressive hemicraniectomy in large hemispherical ischemic strokes. J Stroke Cerebrovasc Dis 27:418–424
Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB (2009) Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 8:326–333
Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW, Gondan M, Schiller P, Limprecht R, Luntz S, Schneider H, Pinzer T, Hobohm C, Meixensberger J, Hacke W (2014) Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med 370:1091–1100
Kurland DB, Khaladj-Ghom A, Stokum JA, Carusillo B, Karimy JK, Gerzanich V, Sahuquillo J, Simard JM (2015) Complications associated with decompressive craniectomy: a systematic review. Neurocrit Care 23:292–304
Kurzbuch AR (2015) Does size matter? Decompressive surgery under review. Neurosurg Rev 38:629–640
Kwak Y, Kim BJ, Park J (2019) Maximum decompressive hemicraniectomy for patients with malignant hemispheric infarction. J Cerebrovasc Endovasc Neurosurg 21:138–143
Müller HP, Unrath A, Ludolph AC, Kassubek J (2007) Preservation of diffusion tensor properties during spatial normalization by use of tensor imaging and fibre tracking on a normal brain database. Phys Med Biol 52:N99-109
Neugebauer H, Creutzfeldt CJ, Hemphill JC 3rd, Heuschmann PU, Jüttler E (2014) DESTINY-S: attitudes of physicians toward disability and treatment in malignant MCA infarction. Neurocrit Care 21:27–34
Neugebauer H, Fiss I, Pinczolits A, Hecht N, Witsch J, Dengler NF, Vajkoczy P, Juttler E, Woitzik J (2016) Large size hemicraniectomy reduces early herniation in malignant middle cerebral artery infarction. Cerebrovasc Dis 41:283–290
Neugebauer H, Heuschmann PU, Juttler E (2012) DEcompressive Surgery for the Treatment of malignant INfarction of the middle cerebral arterY - Registry (DESTINY-R): design and protocols. BMC Neurol 12:115
Neugebauer H, Schnabl M, Lulé D, Heuschmann PU, Jüttler E (2017) Attitudes of patients and relatives toward disability and treatment in malignant MCA infarction. Neurocrit Care 26:311–318
Pinkhardt EH, Issa H, Gorges M, Jürgens R, Lulé D, Heimrath J, Müller HP, Ludolph AC, Becker W, Kassubek J (2014) Do eye movement impairments in patients with small vessel cerebrovascular disease depend on lesion load or on cognitive deficits? A video-oculographic and MRI study. J Neurol 261:791–803
Reinink H, Jüttler E, Hacke W, Hofmeijer J, Vicaut E, Vahedi K, Slezins J, Su Y, Fan L, Kumral E, Greving JP, Algra A, Kappelle LJ, van der Worp HB, Neugebauer H (2020) Surgical decompression for space-occupying hemispheric infarction: a systematic review and individual patient meta-analysis of randomized clinical trials. JAMA Neurol 78(2):208
Shu J, Neugebauer H, Li F, Lulé D, Müller HP, Zhang J, Ludolph AC, Huang Y, Kassubek J, Zhang W (2019) Clinical and neuroimaging disparity between Chinese and German patients with cerebral small vessel disease: a comparative study. Sci Rep 9:20015
Tanrikulu L, Oez-Tanrikulu A, Weiss C, Scholz T, Schiefer J, Clusmann H, Schubert GA (2015) The bigger, the better? About the size of decompressive hemicraniectomies. Clin Neurol Neurosurg 135:15–21
Torbey MT, Bösel J, Rhoney DH, Rincon F, Staykov D, Amar AP, Varelas PN, Jüttler E, Olson D, Huttner HB, Zweckberger K, Sheth KN, Dohmen C, Brambrink AM, Mayer SA, Zaidat OO, Hacke W, Schwab S (2015) Evidence-based guidelines for the management of large hemispheric infarction : a statement for health care professionals from the neurocritical care society and the German society for neuro-intensive care and emergency medicine. Neurocrit Care 22:146–164
Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W (2007) Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 6:215–222
Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, Boutron C, Couvreur G, Rouanet F, Touzé E, Guillon B, Carpentier A, Yelnik A, George B, Payen D, Bousser MG (2007) Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 38:2506–2517
van der Worp HB, Hofmeijer J, Jüttler E, Lal A, Michel P, Santalucia P, Schönenberger S, Steiner T, Thomalla G (2021) European stroke organisation (ESO) guidelines on the management of space-occupying brain infarction. Eur Stroke J 6(2):XC–CX. https://doi.org/10.1177/23969873211014112
von Olnhausen O, Thorén M, von Vogelsang A-C, Svensson M, Schechtmann G (2016) Predictive factors for decompressive hemicraniectomy in malignant middle cerebral artery infarction. Acta Neurochir 158:865–873
Wagner S, Schnippering H, Aschoff A, Koziol JA, Schwab S, Steiner T (2001) Suboptimum hemicraniectomy as a cause of additional cerebral lesions in patients with malignant infarction of the middle cerebral artery. J Neurosurg 94:693–696
Whitwell JL, Crum WR, Watt HC, Fox NC (2001) Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol 22:1483–1489
Wirtz CR, Steiner T, Aschoff A, Schwab S, Schnippering H, Steiner HH, Hacke W, Kunze S (1997) Hemicraniectomy with dural augmentation in medically uncontrollable hemispheric infarction. Neurosurg Focus 2(5):E7
Zahuranec DB, Morgenstern LB, Sánchez BN, Resnicow K, White DB, Hemphill JC (2010) Do-not-resuscitate orders and predictive models after intracerebral hemorrhage. Neurology 75:626–633
Acknowledgements
The authors thank Victoria Rücker, PhD (Institute of Clinical Epidemiology and Biometry, University Würzburg) for her support in data analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors report no targeted funding.
Author information
Authors and Affiliations
Consortia
Contributions
EJ and HN conceived, designed and supervised the study. H-PM and JK analyzed radiologic data. Analyses of clinical data were performed by DL and HN. The first draft of the manuscript was written by DL and all authors commented on previous versions of the manuscript. Data collection was performed by NH, GT, DM, TG, KEW, JS-A, HH, JBK, SW, H-HS, KW, SH, AG, HS, SP, CD, JW, EJ and HN. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
D. Lehrieder, H.P. Müller, J. Kassubek, G. Thomalla, D. Michalski, T. Gattringer, K.E. Wartenberg, J. Schultze-Amberger, H. Huttner, J.B. Kuramatsu, S. Wunderlich, H.H. Steiner, K. Weissenborn, S. Heck, H. Schneider, C. Dohmen, J. Woitzik and H. Neugebauer report no disclosures relevant to the manuscript; N. Hecht is Berlin Institute of Health Clinical Fellow, funded by Stiftung Charité; S. Poli is coordinating investigator of Automatic PredICtion of Edema after Stroke (APICES) study (ClinicalTrials.gov unique identifier: NCT04057690); A. Günther reports honoria from Pfizer, Bristol-Myers-Squibb, Boeringer Ingelheim, Daiichi Sankyo, Occlutech, Ipsen Pharma and Merz outside the submitted work; E. Jüttler reports honoria from Pfizer, Bristol-Myers-Squibb, Boehringer Ingelheim, Daiichi Sankyo and Stryker outside the submitted work. He was Principle Investigator of DESTINY and DESTINY II, Co-Investigator of the pooled analyses of randomized hemicraniectomy trials and Co-author of guidelines on intracranial pressure treatment and treatment of space-occupying brain infarction.
Ethical approval
The trial was registered in the German Registry for Clinical Studies (DRKS00000624). The ethics committee of the Charité – Universitätsmedizin Berlin, Campus Benjamin Franklin (EA4/108/09) and the local ethics committees of all participating centers approved this registry. Written informed consent was obtained from all patients or legal representatives.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lehrieder, D., Müller, HP., Kassubek, J. et al. Large diameter hemicraniectomy does not improve long-term outcome in malignant infarction. J Neurol 270, 4080–4089 (2023). https://doi.org/10.1007/s00415-023-11766-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11766-3