Abstract
Background
No specific treatment has demonstrated its effectiveness to prevent post-partum relapses for multiple sclerosis (MS) women.
Objective
To assess the effectiveness of preventive high-dose corticosteroids in the post-partum period by comparing two strategies: (1) no preventive treatment and (2) standardized preventive treatment.
Methods
We selected five French Multiple Sclerosis centers using the same post-partum strategy for their patients—either high-dose steroids (treating centers TC) or no treatment (non-treating centers NTC). We included relapsing–remitting multiple sclerosis women who delivered between January 2007 and January 2017. Our primary outcomes were the time from delivery to first relapse, EDSS progression and MRI activity between patients of treating centers and non-treating centers, after propensity-score weighting.
Results
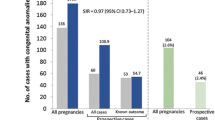
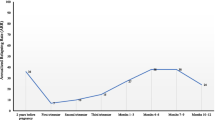
350 patients were included (116 from treating centers, 234 from non-treating centers). For both groups, the annualized relapse rate decreased during pregnancy (0.28 in treating centers and 0.34 in non-treating centers during the third trimester) and increased during the first post-partum trimester (0.45 and 0.69, respectively) with 11% and 14% (NS) of patients facing at least one relapse, respectively. Our primary outcomes were not statistically different between both groups.
Conclusion
This study provides class III evidence that systematic high-dose corticosteroids are not associated with a reduced inflammatory activity during the post-partum period in multiple sclerosis patients.




Similar content being viewed by others

References
Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 339:285–291
Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P et al (2004) Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 127(Pt 6):1353–1360
Vukusic S, Ionescu I, Cornu C, Bossard N, Durand-Dubief F, Cotton F et al (2020) Oral nomegestrol acetate and transdermal 17-beta-estradiol for preventing post-partum relapses in multiple sclerosis: the POPARTMUS study. Mult Scler 27:1458–1463
Hughes SE, Spelman T, Gray OM, Boz C, Trojano M, Lugaresi A et al (2014) Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler 20:739–746
Fragoso YD, Boggild M, Macias-Islas MA, Carra A, Schaerer KD, Aguayo A et al (2013) The effects of long-term exposure to disease-modifying drugs during pregnancy in multiple sclerosis. Clin Neurol Neurosurg 115:154–159
Hellwig K, Haghikia A, Rockhoff M, Gold R (2012) Multiple sclerosis and pregnancy: experience from a nationwide database in Germany. Ther Adv Neurol Disord 5:247–253
Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J (2018) Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology 90:e840–e846
Bsteh G, Algrang L, Hegen H, Auer M, Wurth S, Di Pauli F et al (2020) Pregnancy and multiple sclerosis in the DMT era: a cohort study in Western Austria. Mult Scler 26:69–78
Berenguer-Ruiz L, Gimenez-Martinez J, Palazón-Bru A, Sempere AP (2019) Relapses and obstetric outcomes in women with multiple sclerosis planning pregnancy. J Neurol 266:2512–2517
Finkelsztejn A, Fragoso YD, Ferreira MLB, Lana-Peixoto MA, Alves-Leon SV, Gomes S et al (2011) The Brazilian database on pregnancy in multiple sclerosis. Clin Neurol Neurosurg 113:277–280
Jesus-Ribeiro J, Correia I, Martins AI, Fonseca M, Marques I, Batista S et al (2017) Pregnancy in Multiple Sclerosis: a Portuguese cohort study. Mult Scler Relat Disord 17:63–68
Langer-Gould A, Smith JB, Albers KB, Xiang AH, Wu J, Kerezsi EH et al (2020) Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology 94:e1939–e1949
Vukusic S, Durand-Dubief F, Benoit A, Marignier R, Frangoulis B, Confavreux C (2015) Natalizumab for the prevention of post-partum relapses in women with multiple sclerosis. Mult Scler 21:953–955
Portaccio E, Moiola L, Martinelli V, Annovazzi P, Ghezzi A, Zaffaroni M et al (2018) Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: II: maternal risks. Neurology 90:e832–e839
Canibaño B, Deleu D, Mesraoua B, Melikyan G, Ibrahim F, Hanssens Y (2020) Pregnancy-related issues in women with multiple sclerosis: an evidence-based review with practical recommendations. J Drug Assess 9:20–36
de Seze J, Chapelotte M, Delalande S, Ferriby D, Stojkovic T, Vermersch P (2004) Intravenous corticosteroids in the postpartum period for reduction of acute exacerbations in multiple sclerosis. Mult Scler J 10:596–597
Avila-Ornelas J, Avila M, Stosic M, Robles L, Prieto PG, Hutton GJ et al (2011) The role of postpartum intravenous corticosteroids in the prevention of relapses in multiple sclerosis. Int J MS Care 13:91–93
Hellwig K, Beste C, Schimrigk S, Chan A (2009) Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disord 2:7–11
Brandt-Wouters E, Gerlach OHH, Hupperts RMM (2016) The effect of postpartum intravenous immunoglobulins on the relapse rate among patients with multiple sclerosis. Int J Gynaecol Obstet 134:194–196
Rosa GR, O’Brien AT, de Nogueira EAG, de Carvalho VM, Paz SC, Fragoso YD (2018) There is no benefit in the use of postnatal intravenous immunoglobulin for the prevention of relapses of multiple sclerosis: findings from a systematic review and meta-analysis. Arq Neuropsiquiatr 76:361–366
Horvat Ledinek A, Brecl Jakob G, Jerše J, Ruška B, Pavičić T, Gabelić T et al (2020) Intravenous immunoglobulins for the prevention of postpartum relapses in multiple sclerosis. Mult Scler Relat Disord 38:101519
Winkelmann A, Rommer PS, Hecker M, Zettl UK (2018) Intravenous immunoglobulin treatment in multiple sclerosis: a prospective, rater-blinded analysis of relapse rates during pregnancy and the postnatal period. CNS Neurosci Ther 25:78–85
Portaccio E, Ghezzi A, Hakiki B, Sturchio A, Martinelli V, Moiola L et al (2014) Postpartum relapses increase the risk of disability progression in multiple sclerosis: the role of disease modifying drugs. J Neurol Neurosurg Psychiatry 85:845–850
Vukusic S, Casey R, Rollot F, Brochet B, Pelletier J, Laplaud D-A et al (2020) Observatoire Français de la Sclérose en Plaques (OFSEP): a unique multimodal nationwide MS registry in France. Mult Scler 26:118–122
Confavreux C, Compston DA, Hommes OR, McDonald WI, Thompson AJ (1992) EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry 55:671–676
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T (2006) Variable selection for propensity score models. Am J Epidemiol 163:1149–1156
Karim ME, Pellegrini F, Platt RW, Simoneau G, Rouette J, de Moor C. The use and quality of reporting of propensity score methods in multiple sclerosis literature: a review. Mult Scler. 2020;1352458520972557
Simoneau G, Pellegrini F, Debray TP, Rouette J, Muñoz J, Platt RW et al (2022) Recommendations for the use of propensity score methods in multiple sclerosis research. Mult Scler. https://doi.org/10.1177/13524585221085732
Zhao L, Claggett B, Tian L, Uno H, Pfeffer MA, Solomon SD et al (2016) On the restricted mean survival time curve in survival analysis. Biometrics 72:215–221
RISCA: Causal Inference and Prediction in Cohort-Based Analyses version 0.9 from CRAN. https://rdrr.io/cran/RISCA/. Accessed 26 Feb 2021
Voskuhl RR, Wang H, Wu TCJ, Sicotte NL, Nakamura K, Kurth F et al (2016) Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 15:35–46
Hellwig K, Rockhoff M, Herbstritt S, Borisow N, Haghikia A, Elias-Hamp B et al (2015) Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol 72:1132–1138
Krysko KM, Rutatangwa A, Graves J, Lazar A, Waubant E (2020) Association between breastfeeding and postpartum multiple sclerosis relapses: a systematic review and meta-analysis. JAMA Neurol 77:327–338
Couvrat-Desvergnes G, Foucher Y, Le Borgne F, Dion A, Mourad G, Garrigue V et al (2019) Comparison of graft and patient survival according to the transplantation centre policy for 1-year screening biopsy among stable kidney recipients: a propensity score-based study. Nephrol Dial Transplant 34:703–711
Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C et al (1997) Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry 62:112–118
Acknowledgements
The present study is supported by a grant from the ARSEP. This work has been done with data from the OFSEP (http://www.ofsep.org/fr/http://www.ofsep.org/fr/), which is supported by a grant provided by the French State and handled by the Agence Nationale de la Recherche within the framework of the Investments for the Future program under the reference ANR-10-COHO-002 OFSEP and by the Eugene Devic Foundation Against Multiple Sclerosis. The authors thank the technicians of participating centers for their help (Mr Leport from Rennes, Mrs Moyon from Nantes, Mrs Callier from Nice, Mrs Berthe from Strasbourg). The authors also thank Merck Serono for partly supporting this study.
Funding
This work has been granted by the Eugene Devic EDMUS Foundation against Multiple Sclerosis and by the Aide à la Recherche sur la Sclérose en Plaques (ARSEP) foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LM received honoraria as speaker from Biogen, Merck Serono, BMS Celgene, Sanofi Genzyme, Roche, and Novartis. SV reports consulting and lecture fees, travel grants and research support from Biogen, Celgène, Novartis, Merck, Roche, Sanofi Genzyme and Teva Pharm. DAL has received consulting and lecturing fees, travel grants and unconditional research support from Biogen, Genzyme, Novartis, Merck, Roche, Sanofi, Medday, Teva Pharma and BMS. SW received honoraria as speaker or consultant from Alexion, Biogen, Merck Serono, Roche, and Novartis. ELP received honoraria for lectures or consulting from Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, TEVA, Roche, Alexion. SL, ML, LL, AM, GE, AK, JDS, CLF, EL have no no conflict of interest regarding this work.
Ethical approval
All procedures performed this study were in accordance with the ethical standards of the institutional and national research committee. The present study received approval from the Comité de Protection des Personnes on April 16th 2019 (registration number SI: 19.03.05.63609) and was declared to the Agence Nationale de Sécurité du Médicament et des produits de santé (ID RCB:2019-A00567-50).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leguy, S., Lefort, M., Lescot, L. et al. COPP-MS: COrticosteroids during the Post-Partum in relapsing Multiple Sclerosis patients. J Neurol 269, 5571–5581 (2022). https://doi.org/10.1007/s00415-022-11215-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11215-7