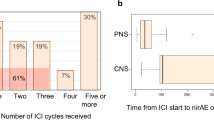
Abstract
As the use of cancer immunotherapy with immune checkpoint inhibitors (ICIs) is expanding rapidly for the treatment of many tumor types, it is crucial that both neurologists and oncologists become familiar with the diagnosis and treatment of neurological immune-related adverse events (n-irAEs). These are rare complications, developing in their severe forms in only 1–3% of the patients, but are highly relevant due to their mortality and morbidity burden. The diagnosis of n-irAEs is—however—challenging, as many alternative diagnoses need to be considered in the complex scenario of a patient with advanced cancer developing neurological problems. A tailored diagnostic approach is advisable according to the presentation, clinical history, and known specificities of n-irAEs. Several patterns characterized by distinct clinical, immunological, and prognostic characteristics are beginning to emerge. For example, myasthenia gravis is more likely to develop after anti-programmed cell death protein 1 (PD-1) or anti-programmed cell death ligand 1 (PD-L1) treatment, while meningitis appears more frequently after anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) therapy. Also, peripheral neuropathy and Guillain–Barré syndrome seem to be more common in patients with an underlying melanoma. Central nervous system disorders (CNS) are less frequent and are more often associated with lung cancer, and some of them (especially those with limbic encephalitis and positive onconeural antibodies) have a poor prognosis. Herein, we provide an update of the recent advances in the diagnosis and treatment of neurological toxicities related to ICI use, focusing on the exclusion of alternative diagnoses, diagnostic specificities, and treatment of n-irAEs.




Similar content being viewed by others
Data access, responsibility, and analysis
The Corresponding Author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Marini A, Bernardini A, Gigli GL et al (2021) Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology 96:754–766. https://doi.org/10.1212/WNL.0000000000011795
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355. https://doi.org/10.1126/science.aar4060
Vaddepally RK, Kharel P, Pandey R et al (2020) Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). https://doi.org/10.3390/cancers12030738
Dubey D, David WS, Reynolds KL et al (2020) Severe neurological toxicity of immune checkpoint inhibitors: growing spectrum. Ann Neurol 87:659–669. https://doi.org/10.1002/ana.25708
Brahmer JR, Abu-Sbeih H, Ascierto PA et al (2021) Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9:e002435. https://doi.org/10.1136/jitc-2021-002435
Mahmood SS, Fradley MG, Cohen JV et al (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71:1755–1764. https://doi.org/10.1016/j.jacc.2018.02.037
Graus F, Vogrig A, Muñiz-Castrillo S et al (2021) Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000001014
Santomasso BD (2020) Anticancer drugs and the nervous system. Continuum (Minneap Minn) 26:732–764. https://doi.org/10.1212/CON.0000000000000873
Lee EQ (2020) Neurologic complications in patients with cancer. Continuum (Minneap Minn) 26:1629–1645. https://doi.org/10.1212/CON.0000000000000937
Pruitt AA (2012) CNS infections in patients with cancer. Continuum (Minneap Minn) 18:384–405. https://doi.org/10.1212/01.CON.0000413665.80915.c4
Le Rhun E, Taillibert S, Chamberlain MC (2013) Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 4:S265-288. https://doi.org/10.4103/2152-7806.111304
Muñiz-Castrillo S, Vogrig A, Honnorat J (2020) Associations between HLA and autoimmune neurological diseases with autoantibodies. Autoimmun Highlights 11:2. https://doi.org/10.1186/s13317-019-0124-6
Chang H, Shin Y-W, Keam B et al (2020) HLA-B27 association of autoimmune encephalitis induced by PD-L1 inhibitor. Ann Clin Transl Neurol 7:2243–2250. https://doi.org/10.1002/acn3.51213
Ali OH, Berner F, Bomze D et al (2019) Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer 107:8–14. https://doi.org/10.1016/j.ejca.2018.11.009
Manson G, Maria ATJ, Poizeau F et al (2019) Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J Immunother Cancer 7:337. https://doi.org/10.1186/s40425-019-0821-8
Vogrig A, Muñiz-Castrillo S, Desestret V et al (2020) Pathophysiology of paraneoplastic and autoimmune encephalitis: genes, infections, and checkpoint inhibitors. Ther Adv Neurol Disord 13:1756286420932797. https://doi.org/10.1177/1756286420932797
Guidon AC, Burton LB, Chwalisz BK et al (2021) Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J Immunother Cancer 9:e002890. https://doi.org/10.1136/jitc-2021-002890
Johnson DB, Manouchehri A, Haugh AM et al (2019) Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. https://doi.org/10.1186/s40425-019-0617-x
Vogrig A, Fouret M, Joubert B et al (2019) Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm 6:e604. https://doi.org/10.1212/NXI.0000000000000604
Vogrig A, Muñiz-Castrillo S, Joubert B et al (2020) Central nervous system complications associated with immune checkpoint inhibitors. J Neurol Neurosurg Psychiatry 91:772–778. https://doi.org/10.1136/jnnp-2020-323055
Zell JA, Chang JC (2005) Neoplastic fever: a neglected paraneoplastic syndrome. Support Care Cancer 13:870–877. https://doi.org/10.1007/s00520-005-0825-4
Coureau M, Meert A-P, Berghmans T, Grigoriu B (2020) Efficacy and toxicity of immune -checkpoint inhibitors in patients with preexisting autoimmune disorders. Front Med (Lausanne) 7:137. https://doi.org/10.3389/fmed.2020.00137
Shelly S, Triplett JD, Pinto MV et al (2020) Immune checkpoint inhibitor-associated myopathy: a clinicoseropathologically distinct myopathy. Brain Commun 2:fcaa181. https://doi.org/10.1093/braincomms/fcaa181
Valencia-Sanchez C, Zekeridou A (2021) Paraneoplastic neurological syndromes and beyond emerging with the introduction of immune checkpoint inhibitor cancer immunotherapy. Front Neurol 12:642800. https://doi.org/10.3389/fneur.2021.642800
Dubey D, David WS, Amato AA et al (2019) Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology 93:e1093–e1103. https://doi.org/10.1212/WNL.0000000000008091
Engelborghs S, Niemantsverdriet E, Struyfs H et al (2017) Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst) 8:111–126. https://doi.org/10.1016/j.dadm.2017.04.007
Vogrig A, Muñiz-Castrillo S, Joubert B et al (2021) Cranial nerve disorders associated with immune checkpoint inhibitors. Neurology 96:e866–e875. https://doi.org/10.1212/WNL.0000000000011340
Déchelotte B, Muñiz-Castrillo S, Joubert B et al (2020) Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000000701
Newey CR, Kinzy TG, Punia V, Hantus S (2018) Continuous electroencephalography in the critically ill: clinical and continuous electroencephalography markers for targeted monitoring. J Clin Neurophysiol 35:325–331. https://doi.org/10.1097/WNP.0000000000000475
Vogrig A, Joubert B, André-Obadia N et al (2019) Seizure specificities in patients with antibody-mediated autoimmune encephalitis. Epilepsia 60:1508–1525. https://doi.org/10.1111/epi.16282
Touat M, Maisonobe T, Knauss S et al (2018) Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 91:e985–e994. https://doi.org/10.1212/WNL.0000000000006124
Johansen A, Christensen SJ, Scheie D et al (2019) Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology 92:663–674. https://doi.org/10.1212/WNL.0000000000007235
Sechi E, Markovic SN, McKeon A et al (2020) Neurologic autoimmunity and immune checkpoint inhibitors: autoantibody profiles and outcomes. Neurology 95:e2442–e2452. https://doi.org/10.1212/WNL.0000000000010632
Moreira A, Loquai C, Pföhler C et al (2019) Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 106:12–23. https://doi.org/10.1016/j.ejca.2018.09.033
Suzuki S, Ishikawa N, Konoeda F et al (2017) Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 89:1127–1134. https://doi.org/10.1212/WNL.0000000000004359
Bourgeois-Vionnet J, Joubert B, Bernard E et al (2018) Nivolumab-induced myositis: a case report and a literature review. J Neurol Sci 387:51–53. https://doi.org/10.1016/j.jns.2018.01.030
Nakatani Y, Tanaka N, Enami T et al (2018) Lambert–Eaton myasthenic syndrome caused by nivolumab in a patient with squamous cell lung cancer. Case Rep Neurol 10:346–352. https://doi.org/10.1159/000494078
Huang Y-T, Chen Y-P, Lin W-C et al (2020) Immune checkpoint inhibitor-induced myasthenia gravis. Front Neurol 11:634. https://doi.org/10.3389/fneur.2020.00634
Mitsune A, Yanagisawa S, Fukuhara T et al (2018) Relapsed myasthenia gravis after nivolumab treatment. Intern Med 57:1893–1897. https://doi.org/10.2169/internalmedicine.9153-17
Patel AS, Snook RJ, Sehdev A (2019) Chronic inflammatory demyelinating polyradiculoneuropathy secondary to immune checkpoint inhibitors in melanoma patients. Discov Med 28:107–111
Okada K, Seki M, Yaguchi H et al (2021) Polyradiculoneuropathy induced by immune checkpoint inhibitors: a case series and review of the literature. J Neurol 268:680–688. https://doi.org/10.1007/s00415-020-10213-x
Clarke JL (2012) Leptomeningeal metastasis from systemic cancer. Continuum (Minneap Minn) 18:328–342. https://doi.org/10.1212/01.CON.0000413661.58045.e7
Taillibert S, Chamberlain MC (2018) Leptomeningeal metastasis. Handb Clin Neurol 149:169–204. https://doi.org/10.1016/B978-0-12-811161-1.00013-X
Nersesjan V, McWilliam O, Krarup L-H, Kondziella D (2021) Autoimmune encephalitis related to cancer treatment with immune checkpoint inhibitors: a systematic review. Neurology 97:e191–e202. https://doi.org/10.1212/WNL.0000000000012122
Mongay-Ochoa N, Vogrig A, Muñiz-Castrillo S, Honnorat J (2020) Anti-Hu-associated paraneoplastic syndromes triggered by immune-checkpoint inhibitor treatment. J Neurol 267:2154–2156. https://doi.org/10.1007/s00415-020-09940-y
Velasco R, Villagrán M, Jové M et al (2021) Encephalitis induced by immune checkpoint inhibitors: a systematic review. JAMA Neurol 78:864–873. https://doi.org/10.1001/jamaneurol.2021.0249
Hébert J, Riche B, Vogrig A et al (2020) Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000000883
Vogrig A, Gigli GL, Segatti S et al (2020) Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol 267:26–35. https://doi.org/10.1007/s00415-019-09544-1
Vogrig A, Joubert B, Maureille A et al (2019) Motor neuron involvement in anti-Ma2-associated paraneoplastic neurological syndrome. J Neurol 266:398–410. https://doi.org/10.1007/s00415-018-9143-x
Graus F (2001) Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 124:1138–1148. https://doi.org/10.1093/brain/124.6.1138
Dalmau J (2004) Clinical analysis of anti-Ma2-associated encephalitis. Brain 127:1831–1844. https://doi.org/10.1093/brain/awh203
Shibaki R, Murakami S, Oki K, Ohe Y (2019) Nivolumab-induced autoimmune encephalitis in an anti-neuronal autoantibody-positive patient. Jpn J Clin Oncol 49:793–794. https://doi.org/10.1093/jjco/hyz087
Matsuoka H, Kimura H, Koba H et al (2018) Nivolumab-induced limbic encephalitis with anti-hu antibody in a patient with advanced pleomorphic carcinoma of the lung. Clin Lung Cancer 19:e597–e599. https://doi.org/10.1016/j.cllc.2018.04.009
Papadopoulos KP, Romero RS, Gonzalez G et al (2018) Anti-hu-associated autoimmune limbic encephalitis in a patient with PD-1 inhibitor-responsive myxoid chondrosarcoma. Oncologist 23:118–120. https://doi.org/10.1634/theoncologist.2017-0344
Duong SL, Barbiero FJ, Nowak RJ, Baehring JM (2021) Neurotoxicities associated with immune checkpoint inhibitor therapy. J Neurooncol 152:265–277. https://doi.org/10.1007/s11060-021-03695-w
Kang JH, Bluestone JA, Young A (2021) Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol 42:293–311. https://doi.org/10.1016/j.it.2021.02.006
Simonaggio A, Michot JM, Voisin AL et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 5:1310. https://doi.org/10.1001/jamaoncol.2019.1022
Dolladille C, Ederhy S, Sassier M et al (2020) Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol 6:865–871. https://doi.org/10.1001/jamaoncol.2020.0726
Allouchery M, Lombard T, Martin M et al (2020) Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥ 2 immune-related adverse events in patients with cancer. J Immunother Cancer 8:e001622. https://doi.org/10.1136/jitc-2020-001622
Mikami T, Liaw B, Asada M et al (2021) Neuroimmunological adverse events associated with immune checkpoint inhibitor: a retrospective, pharmacovigilance study using FAERS database. J Neurooncol 152:135–144. https://doi.org/10.1007/s11060-020-03687-2
Chen X, Schwartz GK, DeAngelis LM et al (2012) Dropped head syndrome: report of three cases during treatment with a MEK inhibitor. Neurology 79:1929–1931. https://doi.org/10.1212/WNL.0b013e318271f87e
Sindoni A, Rodolico C, Pappalardo MA et al (2016) Hypothyroid myopathy: a peculiar clinical presentation of thyroid failure. Review of the literature. Rev Endocr Metab Disord 17:499–519. https://doi.org/10.1007/s11154-016-9357-0
Singer S, Grommes C, Reiner AS et al (2015) Posterior reversible encephalopathy syndrome in patients with cancer. Oncologist 20:806–811. https://doi.org/10.1634/theoncologist.2014-0149
Vogrig A, Zanoni T, Moretto G (2016) Nystagmus and lower extremity hyperalgesia after colectomy. JAMA 316:1488–1489. https://doi.org/10.1001/jama.2016.13658
Bradshaw MJ, Venkatesan A (2016) Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics 13:493–508. https://doi.org/10.1007/s13311-016-0433-7
Funding
This study is supported by FRM (Fondation pour la recherche médicale, DQ20170336751) and has been developed within the BETPSY project, which is supported by a public grant overseen by the French National Research Agency (ANR), as part of the second “Investissements d´Avenir” program (reference ANR-18-RHUS-0012).
Author information
Authors and Affiliations
Contributions
Study concept and design: AV, JH. Acquisition of data: AV, SMC, AF, JH, BJ. Analysis and interpretation of data: AV, SMC, AF, JH, BJ. Drafting of the manuscript: AV, SMC, BJ. Critical revision of the manuscript for important intellectual content: AV, SMC, AF, JH, BJ. Study supervision: JH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Vogrig reports receiving a fellowship grant from the European Academy of Neurology (EAN). No other disclosures were reported.
Rights and permissions
About this article
Cite this article
Vogrig, A., Muñiz-Castrillo, S., Farina, A. et al. How to diagnose and manage neurological toxicities of immune checkpoint inhibitors: an update. J Neurol 269, 1701–1714 (2022). https://doi.org/10.1007/s00415-021-10870-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10870-6