Abstract
The outbreak of a severe acute respiratory syndrome caused by a novel coronavirus (COVID-19), has raised health concerns for patients with multiple sclerosis (MS) who are commonly on long-term immunotherapies. Managing MS during the pandemic remains challenging with little published experience and no evidence-based guidelines. We present five teriflunomide-treated patients with MS who subsequently developed active COVID-19 infection. The patients continued teriflunomide therapy and had self-limiting infection, without relapse of their MS. These observations have implications for the management of MS in the setting of the COVID-19 pandemic.
Similar content being viewed by others
Introduction
In late 2019, a novel enveloped RNA beta-coronavirus (SARS-CoV-2) casing a severe acute respiratory syndrome (COVID-19) emerged in Wuhan, China. In the span of a few months, this outbreak developed into a global pandemic, with a health care crisis, significant mortality rate, and high virulence compared to previously known viral respiratory infections [1]. While the mortality rate in the general population is reported to be approximately 2% (variable by region), individuals with chronic medical conditions and advanced age have even higher mortality [2]. Multiple strategies are under investigation for the prevention and treatment of severe COVID-19, with no vaccine or highly effective therapy currently available [3,4,5,6].
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system that usually requires long-term immunotherapy, and affects more than 1 million Americans [7]. There are currently ~ 20 FDA-approved disease-modifying therapies (DMTs) for the treatment of MS, each resulting in various forms of immunomodulation or immunosuppression. Previous studies have suggested that infections in MS patients are more likely to occur when receiving DMT, require hospitalization more frequently, and are associated with higher mortality than the general public [8,9,10]. Furthermore, systemic infections could trigger disease activity (MS relapse) or worsen pre-existing MS symptoms (pseudo-relapse) [11].
During the emergence of the COVID-19 pandemic, there have been significant health concerns for patients with MS that these medications could increase their risk for acquiring the viral infection, potentially impair antiviral immune responses, and contribute to an unfavorable outcome. COVID-19 poses additional new challenges in managing MS. It has been suggested to discontinue some of the DMTs with highest immunosuppressive capacities during an active COVID-19 infection [12]. However, stopping some DMTs comes with risk of MS rebound or reactivation [13]. Additional concerns relate to the possibility of an inadequate immune response to vaccination in patients on certain DMTs, especially in light of the future potential availability of a SARS-CoV-2vaccine [14]. Thus, managing MS during the COVID-19 pandemic presents a major clinical dilemma and patient reports are now beginning to appear in the literature [15,16,17]. We present five individuals with MS who received oral teriflunomide therapy, developed active COVID-19 infection, continued teriflunomide at the same dose as pre-COVID throughout their infectious illness, and had a favorable outcome (see patient details in Table 1).
Case reports
Patient 1
A 52-year-old man presented in 2017 with a diagnosis of a radiologically isolated demyelinating syndrome, an early stage of MS [18], at which time, teriflunomide was initiated. His other medical conditions included obstructive sleep apnea, hyperlipidemia, attention deficit hyperactivity disorder, and depression treated with atorvastatin, modafinil and fluoxetine. Neurologic examination was normal. MRI, prior to starting DMT, showed a moderate lesion burden and one gadolinium-enhancing lesion, suggesting active MS (Fig. 1). After beginning teriflunomide, he did not experience clinical or MRI worsening. His last available blood examinations (Table 1), from August 2019, showed a decreased white blood cell count of 3.52 K/uL (normal 4–10). On March 20, 2020, he developed severe fatigue, headache, nasal congestion, and loss of smell. Two days later, he developed myalgia, fever, rigors, rhinorrhea, and diarrhea, but no cough, shortness of breath, or sore throat. His residing partner also developed an upper respiratory infection and fever. They both tested positive by nasal swab for COVID-19 infection on 22 March. They self-quarantined at home for 14 days and he was advised by his neurologist to remain on teriflunomide at the same dose. On a neurology virtual visit, 13 days after COVID-19 symptom onset, he reported returning to baseline with no respiratory or neurological symptoms, except a persistent loss of smell. Complete blood count was normal with an ALC of 1.03 K/uL. Twenty-five days after COVID-19 symptom onset, the patient returned to work and reported his smell starting to return to normal.
Patient 1: MRI findings. Representative MRI scans are shown from Patient 1. 3T imaging: in 2017, at the time of initial diagnosis of radiologically isolated syndrome (early multiple sclerosis—MS), fluid-attenuated inversion recovery (upper left, lower middle) showed multiple supratentorial hyperintense white matter lesions, and a pontine hyperintense lesion (arrow), consistent with MS. After gadolinium administration, the left posterior juxtacortical lesion showed enhancement (upper middle, arrow). Right column images show cervical spinal cord hyperintensity (arrows) consistent with MS. 7T imaging: a few years later, T2* imaging demonstrated central (hypointense) veins within a majority of T2 hyperintense lesions (lower left, arrow), highly consistent with MS
Patient 2
A 52-year-old woman with relapsing–remitting MS (RRMS) had neurologic symptom onset in 2002, was treated with teriflunomide since October 2016, and remained stable with mild disability (mild spastic paraparesis and hypoesthesia of the lower limbs). She had no comorbidities and received no other medications. Her last brain MRI from October 2019 was stable (Fig. 2). Her last available blood examinations, from February 20, 2020, are shown in Table 1. On 10 March, the patient developed headaches responsive to paracetamol, associated with nausea and vomiting, mild fever, asthenia, and loss of smell. The patient underwent a nasopharyngeal swab on 15 March that was positive for COVID-19. Due to mild symptomatology, she was asked to self-quarantine at home. She was telephone monitored daily by her neurologist and continued teriflunomide at the same dose. Fifteen days after COVID-19 symptom onset, she showed gradual improvement; by the end of March, she completely recovered. Two follow-up swabs performed 48 h apart (31 March and 2 April) were negative for COVID-19.
Patient 2: MRI findings. The most recent fluid-attenuated inversion-recovery scan is shown from Patient 2, obtained a few months before COVID-19 infection. Note multiple supratentorial hyperintense white matter lesions (right image), and a cerebellar white matter hyperintense lesion (arrow), consistent with multiple sclerosis
Patient 3
A 47-year-old man with RRMS initially presented with myelitis in 2015. He was treated with high dose interferon beta, and then transitioned to teriflunomide (14 mg daily) in 2016 because of breakthrough disease. He had no comorbidities. His exam showed minimal disability with only a mild loss of vibratory sense in the lower extremities. MRI from May 2019 was stable (not shown), and the patient had been doing well. Routine blood examination results from January 2020 are shown in Table 1. On 20 March, he reported a sore throat which had been ongoing for 3 weeks. Two weeks prior, he developed diarrhea and, over the past few days thereafter, developed a low-grade fever, “nagging” cough, and mild dyspnea. Nasal swab from 20 March was positive for COVID-19. His symptoms greatly improved over the following days while he self-quarantined at home. He remained on the same dose of teriflunomide throughout his infection. On 28 April, he reported a return to running 5 miles a day and working full time; a recent chest X-ray was normal.
Patient 4
A 38-year-old man with attention deficit disorder, anxiety, alcohol abuse, and homelessness, was diagnosed with RRMS in 2009. He was on glatiramer acetate for the next 2 years until it was discontinued due to injection site necrosis. He remained untreated by DMT for several years, with clinical relapses involving loss of balance/falls, paresthesias, cognitive decline, and urinary symptoms. He was placed on teriflunomide in November 2019 when MRI of the brain showed a new MS lesion in the corona radiata and the spinal cord showed multiple MS lesions (not shown). Neurologic examination showed mild-moderate overall disability. Routine blood examination from March 2020 is summarized in Table 1. He reported a confirmed diagnosis of COVID-19 on April 16, 2020 with a low-grade fever and dry cough. His symptoms rapidly improved over the following few days without the need for hospitalization; he remained on the same dose of teriflunomide.
Patient 5
This 79-year-old woman initially developed RRMS symptoms in 1992 (left optic neuritis) with exacerbations in 2008 and 2012. She then developed secondary progressive MS (SPMS) characterized by progressive lower extremity weakness, spasticity, and neurogenic bladder, and was switched from interferon beta-1a to teriflunomide (14 mg daily) in September 2017. Her comorbidities included hypertension and recurrent urinary tract infections. After starting teriflunomide, her MS stabilized. In November 2019, LFTs (transaminases) increased to more than ten times normal. After being discontinued for 2 weeks, teriflunomide was restarted at a reduced dose (14 mg every other day). From March 30 to April 2, 2020, she required intensive care hospitalization for Klebsiella pyelonephritis/sepsis but was negative for COVID-19. Teriflunomide was held during this hospitalization for 12 days and restarted on 15 April at 14 mg every other day. On 15 April, the patient developed a dry sore throat and a temperature of 37.7 °C. Nasopharyngeal swab was positive for COVID-19. Blood work from 22 April is shown in Table 1. The patient’s temperature did not exceed 37.5 °C for the 8 days after onset of COVID-19 symptoms. Her COVID-19 symptoms resolved during this time. Teriflunomide was continued at the same dose throughout the course of the COVID-19 illness.
Discussion
Fortunately, a large proportion of patients who develop COVID-19 have self-limiting disease without requiring hospitalization [1, 2]. The poor outcomes related to COVID-19 infection are typically due to pneumonia followed by acute respiratory distress syndrome. Circulatory collapse due to myocarditis and multi-organ failure may also occur. These severe events are characterized by a cytokine storm with fulminant immune activation and direct viral injury to tissue. Both innate and adaptive immunities are involved in the response to these types of viral infections. The activation of the innate immune system is characterized by the production of Type 1 interferons and a resurgence of natural killer cells during the first few days. Interestingly, older non-human primates, when infected with a virus of the same family, exhibited stronger innate inflammatory responses than younger adults, which is accompanied by decreased expression of type I interferon beta [19]. This may be responsible for worse outcomes in older individuals [20]. The adaptive immune response, which includes CD8+ T cells and antibody-producing B cells, is activated a few days after the infection. This provides another line of defense to limit viral replication, remove infected cells, and produce long-term antibody-mediated immunity. Older individuals also have less efficient T- and B-cell responses which could contribute to high mortality rates [21]. As a result, it seems that a delicate balance may be necessary in the host immune response to successfully confront COVID-19 infection. Either overactivation of the innate immune response or an inadequate innate and adaptive immune response could lead to a poor outcome.
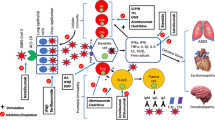
The mechanisms of action of MS immunotherapies span a wide range of targets in the adaptive and innate immune systems. As a result, we speculate on the immunobiologic mechanisms pertaining to teriflunomide’s potential role regarding the favorable outcomes of COVID-19 (Fig. 3). The active metabolite of leflunomide, teriflunomide is an oral FDA-approved DMT shown to limit relapses and the progression of physical disability in patients with relapsing forms of MS [22]. The drug selectively and reversibly inhibits dihydro-orotate dehydrogenase (DHODH), an important mitochondrial enzyme in the de novo pyrimidine synthesis pathway. The downstream effect is reduced proliferation of rapidly dividing cells, including activated T and B lymphocytes, without causing cell death [22]. Owing to the selective targeting of activated immune cells without cell lysis, teriflunomide reduces the level of immune activation without the major immunosuppression that occurs with several other MS DMTs. Thus, one possible beneficial effect of teriflunomide in the face of COVID-19 may be to prevent an excessive/fulminant host immune response, while maintaining an adequate defense against the virus. Furthermore, as we look to the future availability of a vaccine against SARS-CoV-2, it is also helpful to note that teriflunomide-treated patients with MS generally developed effective immunity to seasonal influenza and rabies after these vaccinations [23, 24].
Teriflunomide: relevant potential dual mechanisms of actions. Teriflunomide may express a dual action relevant to active COVID-19 infection. Teriflunomide could potentially interfere with viral replication in infected cells by blocking de novo pyrimidine synthesis and exerting an antiviral effect (left image). In addition, within immune cells (right image), teriflunomide could dampen the unwanted host immune activation through three distinct mechanisms: 1. reducing cytokine production, in particular IL-6, which is thought to contribute to acute respiratory distress syndrome in COVID-19 infection; 2. decreasing immune cell activation by disrupting interactions with antigen-presenting cells (via non-DHOH-mediated impairment of integrin activation and decreased protein aggregation); and 3. blocking cell proliferation through depletion of the intracellular pyrimidine pool
In addition, there is emerging evidence suggesting a direct antiviral effect for teriflunomide and other DHODH inhibitors against a range of viruses such as Theiler’s, virulent Newcastle disease, respiratory syncytial, Ebola, cytomegalovirus, Epstein–Barr, and picornavirus (foot-and-mouth disease) [25,26,27,28]. Moreover, a preliminary pre-clinical study suggests that teriflunomide could combat COVID-19 though dual antiviral and immunomodulatory actions [29]. The proposed main mechanism of antiviral action is depletion of cellular de novo nucleotides biosynthesis, which is required for viral replication (Fig. 3). However, these data should be interpreted with caution, given that there are no clinical studies to date directly demonstrating an antiviral effect of teriflunomide in humans.
Patient 5 had the highest risk of mortality from COVID-19 (due to age and hypertension) and required an intensive care admission for a bacterial infection preceding COVID-19. However, even in the face of these hazards, she recovered completely from COVID-19 while continuing to be treated with teriflunomide. On the other hand, it is important to note that this patient was different from the other four patients, given that she was on a lower (every other day) dose of teriflunomide during the COVID-19 illness and did not receive the drug for 12 days preceding infection. However, given the long half-life of teriflunomide, the drug most likely had carry-over biologic effects in the days immediately preceding the onset of COVID-19 [30].
In conclusion, our observations suggest that teriflunomide may not need to be discontinued in patients with MS who develop an active COVID-19 infection. Furthermore, additional studies may be warranted to assess if the drug can be used to treat COVID-19. However, we must acknowledge that the favorable outcome in our patients may have been unrelated to teriflunomide, given that most patients recover spontaneously without treatment. Moreover, this case series was small, retrospective, open-label, uncontrolled, and non-randomized; future studies are necessary to confirm and extend our preliminary findings.
References
Guan W, Ni Z, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1056/NEJMoa2002032
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Cunningham AC, Goh HP, Koh D (2020) Treatment of COVID-19: old tricks for new challenges. Crit Care 24:91. https://doi.org/10.1186/s13054-020-2818-6
Mahase E (2020) Covid-19: what treatments are being investigated? BMJ 368:m1252. https://doi.org/10.1136/bmj.m1252
Cao B, Wang Y, Wen D et al (2020) A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799. https://doi.org/10.1056/NEJMoa2001282
NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed 15 May 2020
Wallin MT, Culpepper WJ, Campbell JD et al (2019) The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 92:e1029–e1040. https://doi.org/10.1212/WNL.0000000000007035
Luna G, Alping P, Burman J et al (2020) Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 77:184–191. https://doi.org/10.1001/jamaneurol.2019.3365
Karamyan A, Dünser MW, Wiebe DJ et al (2016) Critical illness in patients with multiple sclerosis: a matched case-control study. PLoS ONE 11:e0155795. https://doi.org/10.1371/journal.pone.0155795
Montgomery S, Hillert J, Bahmanyar S (2013) Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol 20:1153–1160. https://doi.org/10.1111/ene.12130
Steelman AJ (2015) Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front Immunol 6:520. https://doi.org/10.3389/fimmu.2015.00520
Giovannoni G, Hawkes C, Lechner-Scott J et al (2020) The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord 39:102073. https://doi.org/10.1016/j.msard.2020.102073
Barry B, Erwin AA, Stevens J, Tornatore C (2019) Fingolimod rebound: a review of the clinical experience and management considerations. Neurol Ther 8:241–250. https://doi.org/10.1007/s40120-019-00160-9
Kim W, Kim S-H, Huh S-Y et al (2013) Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur J Neurol 20:975–980. https://doi.org/10.1111/ene.12132
Sormani MP (2020) An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. https://doi.org/10.1016/S1474-4422(20)30147-2[Epub ahead of print, April 30, 2020]
Novi G, Mikulska M, Briano F et al (2020) COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role. Mult Scler Relat Disord. https://doi.org/10.1016/j.msard.2020.102120[Epub ahead of print, April 15, 2020]
Barzegar M, Mirmosayyeb O, Nehzat N et al (2020) COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm 7:e753. https://doi.org/10.1212/NXI.0000000000000753
Lebrun C (2015) The radiologically isolated syndrome. Rev Neurol (Paris) 171:698–706. https://doi.org/10.1111/jon.12182
Shi Y, Wang Y, Shao C et al (2020) COVID-19 infection: the perspectives on immune responses. Cell Death Differ 27:1451–1454. https://doi.org/10.1038/s41418-020-0530-3
Smits SL, de Lang A, van den Brand JMA et al (2010) Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog 6:e1000756. https://doi.org/10.1371/journal.ppat.1000756
Pinti M, Appay V, Campisi J et al (2016) Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol 46:2286–2301
Bar-Or A, Pachner A, Menguy-Vacheron F et al (2014) Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 74:659–674. https://doi.org/10.1007/s40265-014-0212-x
Bar-Or A, Freedman MS, Kremenchutzky M et al (2013) Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 81:552–558. https://doi.org/10.1212/WNL.0b013e31829e6fbf
Bar-Or A, Wiendl H, Miller B et al (2015) Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens. Neurol Neuroimmunol NeuroInflamm 2:e70. https://doi.org/10.1212/NXI.0000000000000070
Mei-jiao G, Shi-fang L, Yan-yan C et al (2019) Antiviral effects of selected IMPDH and DHODH inhibitors against foot and mouth disease virus. Biomed Pharmacother 118:1–7. https://doi.org/10.1016/j.biopha.2019.109305
Gilli F, Li L, Royce DB et al (2017) Treatment of Theiler’s virus-induced demyelinating disease with teriflunomide. J Neurovirol 23:825–838. https://doi.org/10.1007/s13365-017-0570-8
Bilger A, Plowshay J, Ma S et al (2017) Leflunomide/teriflunomide inhibit Epstein-barr virus (EBV)-induced lymphoproliferative disease and lytic viral replication. Oncotarget 8:44266–44280. https://doi.org/10.18632/oncotarget.17863
Martin S, Chiramel AI, Schmidt ML et al (2018) A genome-wide siRNA screen identifies a druggable host pathway essential for the Ebola virus life cycle. Genome Med 10:58. https://doi.org/10.1186/s13073-018-0570-1
Xiong R, Zhang L, Li S et al (2020) Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. BioRxiv. https://doi.org/10.1101/2020.03.11.983056
Warnke C, Stüve O, Kieseier BC (2013) Teriflunomide for the treatment of multiple sclerosis. Clin Neurol Neurosurg 115:90–94. https://doi.org/10.1016/j.clineuro.2013.09.030
Funding
AH Maghzi is supported by a clinician-scientist development fellowship from the National Multiple Sclerosis Society and American Brain Foundation.
Author information
Authors and Affiliations
Contributions
RB, MKH, and AHM contributed to the conceptualization, gathering of data, drafting, and revising the manuscript. PP, CI, BDB, IB, THWS, AC, and MF contributed to data gathering, drafting, and revising the manuscript. JMS, ST, JAS, MSF, and HLW contributed to drafting and revising the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
MK Houtchens has received consulting fees from Biogen, EMD Serono, Sanofi-Genzyme, Mallinckrodt, Roche and research support from Biogen, EMD Serono, and Sanofi-Genzyme. P Preziosa has received speaking fees from Biogen, ExceMED, Merck Serono, and Novartis. C Ionete has received consulting fees from Sanofi-Genzyme, and research support from Biogen, EMD Serono, and Genentech. JM Stankiewicz has received consulting fees from Biogen, BMS/Celgene, EMD Serono, Genentech, Sanofi-Genzyme, and Novartis. A Cabot has received speaking fees from Alexion, Biogen, EMD/Serono, Genentech, Novartis, and Sanofi-Genzyme. JA Sloane has received consulting fees from Biogen, BMS/Celgene, EMD Serono, Genentech, Alexion, and Sanofi-Genzyme, and research support from National MS Society, Genentech, Biogen, EMD Serono, and Sanofi-Genzyme. MS Freedman has received research or educational grants from EMD Serono, Hoffman-La Roche, and Sanofi-Genzyme, and honoraria, consultation, or speaking fees from Actelion, Alexion, Atara, Bayer, Biogen, BMS/Celgene, Clene Nanomedicine, EMD Serono, GRI Bio, Hoffman La-Roche, Magenta Therapeutics, MedDay, Merck Serono, Novartis, Sanofi-Genzyme, and Teva. M Filippi has received consulting and/or speaking fees from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva, and research support from Biogen, Merck-Serono, Novartis, Roche, and Teva. HL Weiner has received consulting fees from Biodextris, Biogen, CBridge Capital, Everest Medicines, Genentech, Tiziana, Tilos, IM Therapeutics, Magnolia, MedDay, and vTv, and research support from Biogen, EMD Serono, Genentech, Sanofi-Genzyme, Google, Novartis; Teva, Tilos, and Verily. R Bakshi has received consulting fees from Bayer, Biogen, BMS/Celgene, EMD Serono, Genentech, and Novartis, and research support from BMS/Celgene, EMD Serono, and Sanofi-Genzyme. The other authors have nothing to disclose.
Ethical approval
All patients provided consent to be anonymously included in this report.
Informed consent
All authors have consented to publication.
Rights and permissions
About this article
Cite this article
Maghzi, A.H., Houtchens, M.K., Preziosa, P. et al. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J Neurol 267, 2790–2796 (2020). https://doi.org/10.1007/s00415-020-09944-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09944-8