Abstract
Objectives
To compare the efficacy of natalizumab (NTZ) and fingolimod (FTY) in the treatment of relapsing–remitting multiple sclerosis (MS) in sequential use in common and as a function of transition periods in a nationwide observational cohort using prospectively collected data from a real-life setting.
Materials and methods
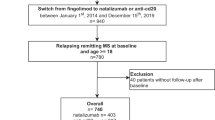
We included 195 patients from the Austrian MS Treatment Registry, who had started treatment with NTZ at any time since 2006 and stayed on NTZ for at least 24 months, switched afterwards within 1 year to FTY and stayed on FTY for at least another 12 months. Transition periods between NTZ and FTY were grouped into three different intervals: < 3 months (135 patients), 3–6 months (44 patients), and 6–12 months (16 patients).
Results
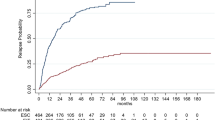
Estimated mean annualized relapse rates (ARR) over a mean treatment period of 44 months were 0.26 for NTZ and 0.32 for FTY (p = 0.381) over 46 months. In the treatment gap, differences were found concerning the relapse probability, seven (5.2%) patients in the < 3 months group, six (13.6%) in thef 3–6 months group, and seven (43.8%) in the 6–12 months group (p < 0.001). After this treatment gap, no significant differences concerning ARR, EDSS change, EDSS progression, and regression were observed regardless the proceeding transition periods. Significantly higher efficacy of NTZ compared to FTY in sequential use was found regarding EDSS change, EDSS progression, and EDSS regression sustained for 12 and 24 weeks.
Conclusions
First, we here show an increased short-time risk for relapses during the treatment gap between NTZ and FTY therapy, dependent on the length of transition time. Second, the disease course after switching to FTY remained stable in the long-term evaluation. Therefore, switching from NTZ to FTY in a real-world setting appears efficacious and safe, but this data advocate for a short switching gap of 3 months or less.



Similar content being viewed by others
Change history
17 September 2019
The original version of this article unfortunately contained a mistake. First and last names of the authors were interchanged. The correct author names are given below.
References
Polman CH, O’Connor PW, Havrdova E et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Kappos L, Radue EW, O’Connor P et al (2010) A placebo- controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Calabresi PA, Radue EW, Goodin D et al (2014) Safety and efficacy of fingolimod in patients with relapsing- remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13:545–556
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Plavina T, Subramanyam M, Bloomgren G et al (2014) Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 76(6):802–812
O’Connor PW, Goodman A, Kappos L et al (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76:1858–1865
Salhofer-Polanyi S, Baumgartner A, Kraus J et al (2014) What to expect after natalizumab cessation in a real-life setting. Acta Neurol Scand 130:97–102
Cohen M, Maillart E, Tourbah A et al (2014) Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol 71:436–441
Diem L, Nedeltchev K, Kahles T et al (2018) Long-term evaluation of NEDA-3 status in relapsing-remitting multiple sclerosis patients after switching from natalizumab to fingolimod. Ther Adv Neurol Disord. 9(11):1756286418791103
Comi G, Gold R, Dahlke F et al (2015) Relapses in patients treated with fingolimod after previous exposure to natalizumab. Mult Scler 21:786–790
Sempere AP, Martin-Medina P, Berenguer- Ruiz L et al (2013) Switching from natalizumab to fingolimod: an observational study. Acta Neurol Scand 128:e6–e10
Havla J, Tackenberg B, Hellwig K et al (2013) Fingolimod reduces recurrence of disease activity after natalizumab withdrawal in multiple sclerosis. J Neurol 260:1382–1387
Jokubaitis VG, Li V, Kalincik T, Izquierdo G (2014) Fingolimod after natalizumab and the risk of short-term relapse. Neurology. 82(14):1204–1211
Villaverde-Gonzalez R, Gracia Gil J, Perez Sempere A et al (2017) Observational study of switching from natalizumab to immunomodulatory drugs. Eur Neurol 77:130–136
Vollmer B, Honce JM, Sillau S et al (2018) The impact of very short transition times on switching from Natalizumab to Fingolimod on imaging and clinical effectiveness outcomes in multiple sclerosis. J Neurol Sci. 15(390):89–93
Leurs CE, van Kempen ZL, Dekker I et al (2018) Switching natalizumab to fingolimod within 6 weeks reduces recurrence of disease activity in MS patients. Mult Scler. 24(11):1453–1460
Guger M, Enzinger C, Leutmezer F et al (2018) Real-life clinical use of natalizumab and fingolimod in Austria. Acta Neurol Scand. 137(2):181–187
Kerbrat A, Le Page E, Leray E et al (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J. Neurol. Sci. 308(1):98–102
Giovannoni G, Marta M, Davis A et al (2016) Switching patients at high risk of PML from natalizumab to another disease-modifying therapy. Pract. Neurol. 16(5):389
Acknowledgements
The Steering Group wishes to thank all Austrian MS centres (https://www.oegn.at/neurologie-in-oesterreich/ms-zentren/) for contributing data to the registry and to the patients for providing written informed consent.
Funding
The Austrian MS Treatment Registry is supported by unrestricted grants of Biogen Austria, Novartis Pharma Austria and Genzyme Austria.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Michael Guger received support and honoraria for research, consultation, lectures, and education from Almirall, Bayer, Biogen, Celgene, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi Aventis, Shire, and TEVA ratiopharm. Christian Enzinger received funding for travel and speaker honoraria from Biogen, Bayer, Celgene, Merck, Novartis, Roche, Shire, Sanofi-Genzyme, and Teva Pharmaceutical Industries Ltd./sanofi-aventis, research support from Merck Serono, Biogen, Sanofi-Genzyme, and Teva Pharmaceutical Industries Ltd./sanofi-aventis and serving on scientific advisory boards for Bayer Schering, Biogen, Merck Serono, Novartis, Roche, and Teva Pharmaceutical Industries Ltd./sanofi-aventis. Fritz Leutmezer has received funding for travel and speaker honoraria from Biogen, Bayer Schering Pharma, Merck Serono, Novartis, Genzyme, Santhera, and Teva Pharmaceutical Industries Ltd./sanofi-aventis. Jörg Kraus received consulting and/or research funding and/or educational support from Almirall, Bayer, Biogen, Celgene, MedDay, Medtronic, Merck, Novartis, Roche, Sanofi-Aventis, Shire, TEVA ratiopharm. Stefan Kalcher declares that there is no conflict of interest. Erich Kvas declares that there is no conflict of interest. Thomas Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Bayer, Biogen, Biologix, Bionorica, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme, TG Pharmaceuticals, TEVA-ratiopharm, and UCB. His institution has received financial support in the last 12 months by unrestricted research grants (Biogen, Bayer, Merck, Novartis, Sanofi/Genzyme, and TEVA ratiopharm) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme, and TEVA.
Ethical standards
The AMSTR is compliant with Austrian laws on bioethics and it was also approved by the ethical committee of the Medical University of Vienna (EC number 2096/2013).
Additional information
The original version of this article was revised: First and last names of the authors were interchanged.
Rights and permissions
About this article
Cite this article
Guger, M., Enzinger, C., Leutmezer, F. et al. Switching from natalizumab to fingolimod treatment in multiple sclerosis: real life data from the Austrian MS Treatment Registry. J Neurol 266, 2672–2677 (2019). https://doi.org/10.1007/s00415-019-09464-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09464-0