Abstract
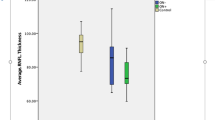
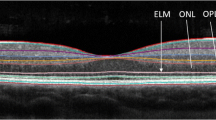
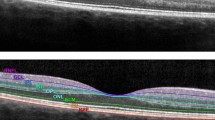
The aim of this study was to summarise existing findings regarding optical coherence tomography (OCT) measurements of ganglion cell layer (GCL) alterations in optic neuritis (ON) and multiple sclerosis (MS). Peer-reviewed studies published prior to April 2016 were searched using PubMed, EMBASE, Web of Science and Scopus. Studies were included if they measured GCL thickness using OCT in patients with either ON, MS or clinically isolated syndrome. For the meta-analysis, we compared GCL thickness in MS patients with and without prior ON, to healthy controls. 42/252 studies were reviewed. In acute ON, studies showed significant thinning of the GCL within the first 5 weeks (n = 5), earlier than retinal nerve fibre layer (RNFL) thinning. GCL thinning at 1–2 months after acute ON predicted visual function at 6 months (n = 3). The meta-analysis showed that the thickness of the GCL was significantly reduced in MS patients both with and without previous ON compared to healthy controls. GCL thinning was associated with visual function in most studies (n = 10) and expanded disability status scale (EDSS) scores (n = 6). In acute ON, thinning of the GCL is measurable prior to RNFL thinning, and GCL thickness after 1–2 months may predict visual function after 6 months. Furthermore, GCL thinning occurs in MS both with and without prior ON, and may be associated with visual function and EDSS score. This suggests that the GCL is a promising biomarker, which may be used to examine in vivo neurodegeneration in ON and MS.





Similar content being viewed by others
References
Petzold A, Wattjes MP, Costello F et al (2014) The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol 10:447–458. doi:10.1038/nrneurol.2014.108
Optic Neuritis Study Group (2008) Multiple Sclerosis Risk After Optic Neuritis. Arch Neurol 65:727–732. doi:10.1001/archneur.65.6.727
Frohman EM, Fujimoto JG, Frohman TC et al (2008) Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 4:664–675. doi:10.1038/ncpneuro0950
Perry VH, Lund RD (1990) Evidence that the lamina cribrosa prevents intraretinal myelination of retinal ganglion cell axons. J Neurocytol 19:265–272
Ikuta F, Zimmerman HM (1976) Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology 26:26–28
Toussaint D, Périer O, Verstappen A, Bervoets S (1983) Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol 3:211–220
Petzold A, de Boer JF, Schippling S et al (2010) Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 9:921–932. doi:10.1016/S1474-4422(10)70168-X
Kupersmith MJ, Mandel G, Anderson S et al (2011) Baseline, one and three month changes in the peripapillary retinal nerve fiber layer in acute optic neuritis: relation to baseline vision and MRI. J Neurol Sci 308:117–123. doi:10.1016/j.jns.2011.05.039
Kallenbach K, Simonsen H, Sander B et al (2010) Retinal nerve fiber layer thickness is associated with lesion length in acute optic neuritis. Neurology 74:252–258. doi:10.1212/WNL.0b013e3181ca0135
Wolf-Schnurrbusch UEK, Ceklic L, Brinkmann CK et al (2009) Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci 50:3432–3437. doi:10.1167/iovs.08-2970
Huang D, Swanson EA, Lin CP et al (1991) Optical coherence tomography. Science 254:1178–1181
Balk LJ, Steenwijk MD, Tewarie P et al (2015) Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry 86:419–424. doi:10.1136/jnnp-2014-308189
Balk LJ, Twisk JWR, Steenwijk MD et al (2014) A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry 85:782–789. doi:10.1136/jnnp-2013-306902
Balk L, Tewarie P, Killestein J et al (2014) Disease course heterogeneity and OCT in multiple sclerosis. Mult Scler 20:1198–1206. doi:10.1177/1352458513518626
Seigo MA, Sotirchos ES, Newsome S et al (2012) In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol 259:2119–2130. doi:10.1007/s00415-012-6466-x
Sotirchos ES, Seigo MA, Calabresi PA, Saidha S (2013) Comparison of point estimates and average thicknesses of retinal layers measured using manual optical coherence tomography segmentation for quantification of retinal neurodegeneration in multiple sclerosis. Curr Eye Res 38:224–228. doi:10.3109/02713683.2012.722243
Modvig S, Degn M, Sander B et al (2015) Cerebrospinal fluid neurofilament light chain levels predict visual outcome after optic neuritis. Mult Scler. doi:10.1177/1352458515599074
Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E et al (2015) Dynamics of retinal injury after acute optic neuritis. Ann Neurol 77:517–528. doi:10.1002/ana.24351
Kupersmith MJ, Garvin MK, Wang J-K et al (2015) Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. doi:10.1177/1352458515598020
Behbehani R, Al-Moosa A, Sriraman D, Alroughani R (2016) Ganglion cell analysis in acute optic neuritis. Mult Scler Relat Disord 5:66–69. doi:10.1016/j.msard.2015.10.008
Syc SB, Saidha S, Newsome SD et al (2012) Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 135:521–533. doi:10.1093/brain/awr264
Al-Louzi OA, Bhargava P, Newsome SD et al (2015) Outer retinal changes following acute optic neuritis. Mult Scler. doi:10.1177/1352458515590646
Costello F, Pan YI, Yeh EA et al (2015) The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2014-309704
Schneider E, Zimmermann H, Oberwahrenbrock T et al (2013) Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One 8:e66151. doi:10.1371/journal.pone.0066151
Garcia-Martin E, Polo V, Larrosa JM et al (2014) Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology 121:573–579. doi:10.1016/j.ophtha.2013.09.035
Tegetmeyer H, Kühn E (2011) Quantitative analysis of changes in macular layers following optic neuritis. Neuro-Ophthalmol 35:101–107
Esen E, Sizmaz S, Balal M et al (2016) Evaluation of the innermost retinal layers and visual evoked potentials in patients with multiple sclerosis. Curr Eye Res. doi:10.3109/02713683.2015.1119283
Fernandes DB, Raza AS, Nogueira RGF et al (2013) Evaluation of inner retinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology 120:387–394. doi:10.1016/j.ophtha.2012.07.066
González-López JJ, Rebolleda G, Leal M et al (2014) Comparative diagnostic accuracy of ganglion cell-inner plexiform and retinal nerve fiber layer thickness measures by Cirrus and Spectralis optical coherence tomography in relapsing–remitting multiple sclerosis. Biomed Res Int 2014:128517. doi:10.1155/2014/128517
Hokazono K, Raza AS, Oyamada MK et al (2013) Pattern electroretinogram in neuromyelitis optica and multiple sclerosis with or without optic neuritis and its correlation with FD-OCT and perimetry. Doc Ophthalmol 127:201–215. doi:10.1007/s10633-013-9401-2
Huang-Link YM, Fredrikson M, Link H (2015) Benign multiple sclerosis is associated with reduced thinning of the retinal nerve fiber and ganglion cell layers in non-optic-neuritis eyes. J Clin Neurol 11:241–247. doi:10.3988/jcn.2015.11.3.241
Kaushik M, Wang CY, Barnett MH et al (2013) Inner nuclear layer thickening is inversely proportional to retinal ganglion cell loss in optic neuritis. PLoS One 8:e78341. doi:10.1371/journal.pone.0078341
Klistorner A, Sriram P, Vootakuru N et al (2014) Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology 82:2165–2172. doi:10.1212/WNL.0000000000000522
Narayanan D, Cheng H, Bonem KN et al (2014) Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler 20:1331–1341. doi:10.1177/1352458514523498
Oberwahrenbrock T, Ringelstein M, Jentschke S et al (2013) Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler 19:1887–1895. doi:10.1177/1352458513489757
Petracca M, Cordano C, Cellerino M et al (2016) Retinal degeneration in primary-progressive multiple sclerosis: a role for cortical lesions? Mult Scler. doi:10.1177/1352458516637679
Saidha S, Syc SB, Durbin MK et al (2011) Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler 17:1449–1463. doi:10.1177/1352458511418630
Soufi G, AitBenhaddou E, Hajji Z et al (2015) Evaluation of retinal nerve fiber layer thickness measured by optical coherence tomography in Moroccan patients with multiple sclerosis. J Fr Ophtalmol 38:497–503. doi:10.1016/j.jfo.2014.11.008
Tátrai E, Simó M, Iljicsov A et al (2012) In vivo evaluation of retinal neurodegeneration in patients with multiple sclerosis. PLoS One 7:e30922. doi:10.1371/journal.pone.0030922
Varga BE, Gao W, Laurik KL et al (2015) Investigating tissue optical properties and texture descriptors of the retina in patients with multiple sclerosis. PLoS One 10:e0143711. doi:10.1371/journal.pone.0143711
Walter SD, Ishikawa H, Galetta KM et al (2012) Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 119:1250–1257. doi:10.1016/j.ophtha.2011.11.032
Xu LT, Bermel RA, Nowacki AS, Kaiser PK (2016) Optical coherence tomography for the detection of remote optic neuritis in multiple sclerosis. J Neuroimaging. doi:10.1111/jon.12326
Zimmermann H, Freing A, Kaufhold F et al (2013) Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler 19:443–450. doi:10.1177/1352458512457844
Sriram P, Wang C, Yiannikas C et al (2014) Relationship between optical coherence tomography and electrophysiology of the visual pathway in non-optic neuritis eyes of multiple sclerosis patients. PLoS One 9:e102546. doi:10.1371/journal.pone.0102546
Davies EC, Galetta KM, Sackel DJ et al (2011) Retinal ganglion cell layer volumetric assessment by spectral-domain optical coherence tomography in multiple sclerosis: application of a high-precision manual estimation technique. J Neuroophthalmol 31:260–264. doi:10.1097/WNO.0b013e318221b434
Outteryck O, Majed B, Defoort-Dhellemmes S et al (2015) A comparative optical coherence tomography study in neuromyelitis optica spectrum disorder and multiple sclerosis. Mult Scler. doi:10.1177/1352458515578888
Sriram P, Graham SL, Wang C et al (2012) Transsynaptic retinal degeneration in optic neuropathies: optical coherence tomography study. Invest Ophthalmol Vis Sci 53:1271–1275. doi:10.1167/iovs.11-8732
Albrecht P, Ringelstein M, Müller AK et al (2012) Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler 18:1422–1429. doi:10.1177/1352458512439237
Balk L, Mayer M, Uitdehaag BMJ, Petzold A (2013) Physiological variation of segmented OCT retinal layer thicknesses is short-lasting. J Neurol 260:3109–3114. doi:10.1007/s00415-013-7097-6
Garcia-Martin E, Calvo B, Malvè M et al (2013) Three-dimensional geometries representing the retinal nerve fiber layer in multiple sclerosis, optic neuritis, and healthy eyes. Ophthalmic Res 50:72–81. doi:10.1159/000350413
Droby A, Panagoulias M, Albrecht P et al (2015) A novel automated segmentation method for retinal layers in OCT images proves retinal degeneration after optic neuritis. Br J Ophthalmol. doi:10.1136/bjophthalmol-2014-306015
Saidha S, Sotirchos ES, Oh J et al (2013) Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol 70:34–43. doi:10.1001/jamaneurol.2013.573
Ratchford JN, Saidha S, Sotirchos ES et al (2013) Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 80:47–54. doi:10.1212/WNL.0b013e31827b1a1c
Saidha S, Al-Louzi O, Ratchford JN et al (2015) Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 78:801–813. doi:10.1002/ana.24487
Saidha S, Sotirchos ES, Ibrahim MA et al (2012) Microcystic macular oedema, Thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 11:963–972. doi:10.1016/S1474-4422(12)70213-2
Lampert EJ, Andorra M, Torres-Torres R et al (2015) Color vision impairment in multiple sclerosis points to retinal ganglion cell damage. J Neurol 262:2491–2497. doi:10.1007/s00415-015-7876-3
Álvarez-Cermeño JC, Muñoz-Negrete FJ, Costa-Frossard L et al (2016) Intrathecal lipid-specific oligoclonal IgM synthesis associates with retinal axonal loss in multiple sclerosis. J Neurol Sci 360:41–44. doi:10.1016/j.jns.2015.11.030
Oberwahrenbrock T, Balk L, Petzold A et al (2014) Re: Garcia-Martin et al.: retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography (Ophthalmology 2014;121:573–9). Ophthalmology. doi:10.1016/j.ophtha.2014.06.018
Kimbrough DJ, Sotirchos ES, Wilson JA et al (2015) Retinal damage and vision loss in African American multiple sclerosis patients. Ann Neurol 77:228–236. doi:10.1002/ana.24308
Tewarie P, Balk L, Costello F et al (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 7:e34823. doi:10.1371/journal.pone.0034823
Schippling S, Balk LJ, Costello F et al (2015) Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler 21:163–170. doi:10.1177/1352458514538110
Bhargava P, Lang A, Al-Louzi O et al (2015) Applying an open-source segmentation algorithm to different OCT devices in multiple sclerosis patients and healthy controls: implications for clinical trials. Mult Scler Int 136295. doi:10.1155/2015/136295
Park K-A, Kim J, Oh SY (2014) Analysis of spectral domain optical coherence tomography measurements in optic neuritis: differences in neuromyelitis optica, multiple sclerosis, isolated optic neuritis and normal healthy controls. Acta Ophthalmol 92:e57–e65. doi:10.1111/aos.12215
Acknowledgements
The authors thank Anna Magnussen for illustrations and Jun He for translation from Chinese.
Author information
Authors and Affiliations
Contributions
JB carried out the literature search, systematic review and meta-analysis, and wrote the first draft of the manuscript. GPJ contributed with long-term experience with OCT, statistical guidance and thoroughly revised the manuscript. JLF conceived the idea for this review, made a structure for the manuscript and thoroughly revised the manuscript.
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest
The authors declare that they have no conflicts of interest relevant to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Britze, J., Pihl-Jensen, G. & Frederiksen, J.L. Retinal ganglion cell analysis in multiple sclerosis and optic neuritis: a systematic review and meta-analysis. J Neurol 264, 1837–1853 (2017). https://doi.org/10.1007/s00415-017-8531-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8531-y