Abstract
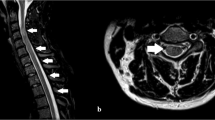
The term “acute transverse myelitis (ATM)” comprises various non-traumatic disorders that eventually can be associated with a focal myelopathy. Patients characteristically present with an acutely occurring paraparesis/plegia and require a comprehensive and timely diagnostic work up for the initiation of an appropriate treatment. We present a case of a 36-year-old female patient with a rare genetic disorder (ANE1: Acute Necrotizing Encephalopathy due to a RANBP2 mutation) who presented with an acute quadriplegia. Following an acute pulmonal infection, she rapidly (< 24 h) developed a severe quadriplegia (total motor score 38) with some facial sensory symptoms (perioral hypoesthesia). Magnetic resonance imaging (MRI) revealed a combination of longitudinal extensive transverse myelitis and symmetrical thalamic lesions. A work-up for infectious and systemic diseases was negative; specifically, no findings related to multiple sclerosis, neuromyelitis optica or vascular disorders. After empirical high dose steroid treatment and rehabilitation therapy, the patient gained almost normal gait and upper limb function. She was found to carry an autosomal-dominant missense mutation in the RANBP2 gene predisposing for ANE. Gene segregation was confirmed in other family members that had been affected by other episodes of acute steroid-responsive encephalopathies. We propose that a redefined diagnostic workup of ATM might include ANE1, as the frequency of this rare disorder might be underestimated.





Similar content being viewed by others
References
Miller FG, Ross AG (1931) Acute transverse myelitis complicating measles. Can Med Assoc J 25(6):709–710
Ropper AH, Poskanzer DC (1978) The prognosis of acute and subacute transverse myelopathy based on early signs and symptoms. Ann Neurol 4(1):51–59
Berman M, Feldman S, Alter S et al (1981) Acute transverse myelitis: incidence and etiologic considerations. Neurology 31:966–971
Jeffery DR, Mandler RN, Davis LE (1993) Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol 50:532–535
Transverse Myelitis Consortium Group (2002) Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 59:499–505
de Seze J, Stojkovic T, Breteau G et al (2001) Acute myelopathies: clinical, laboratory and outcome profiles in 79 cases. Brain 124:1509–1521
Frohman EM, Wingerchuk DM (2010) Transverse myelitis. N Engl J Med 363:564–572
Schmalstieg WF, Weinshenker BG (2010) Approach to acute or subacute myelopathy. Neurology 75:2–8
Neilson DE, Adams MD, Orr C et al (2009) Infection-triggered familial or recurrent cases of Acute Necrotizing Encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet 84:44–51
Krishnan C, Kaplin AI, Deshpande DM et al (2004) Transverse myelitis: pathogenesis, diagnosis and treatment. Front Biosci 9:1483–1499
Scott TF, Kassab SL, Singh S (2005) Acute partial transverse myelitis with normal cerebral magnetic resonance imaging: transition rate to clinically definite multiple sclerosis. Mult Scler 11:373–377
Young J, Quinn S, Hurrel M et al (2009) Clinically isolated acute transverse myelitis: prognostic features and incidence. Mult Scler 15:1295–1302
Klein NP, Ray P, Carpenter D et al (2010) Rates of autoimmune diseases in Kaiser Permanent for use in vaccine adverse event safety studies. Vaccine 28:1062–1068
Pidcock FS, Krishnan C, Crawford TO et al (2007) Acute transverse myelitis in childhood: center-based analysis of 47 cases. Neurology 68:1474–1480
Banwel B, Kennedy J, Sadovnick D et al (2009) Incidence of acquired demyelination of the CNS in Canadian children. Neurology 72:232–239
de Seze J, Lanctin C, Lebrun C et al (2005) Ideopathic acute transverse myelitis: application of the recent diagnostic criteria. Neurology 65:1950–1953
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic Criteria for multiple sclerosis: 2010 Revision of the McDonald criteria. Ann Neurol 69:292–302
Borchers AT, Gershwin ME (2012) Transverse myelitis. Autoimmun Rev 11:231–248
Scott TF (2007) Nosology of ideopathic transverse myelitis syndromes. Acta Neurol Scand 115:371–376
Sellner J, Luthi N, Buhler R et al (2008) Acute partial transverse myelitis: risk factors for conversion to multiple sclerosis. Eur J Neurol 15:398–405
Cordonnier C, de Seze J, Breteau G et al (2003) Prospective study of patients presenting with acute partial transverse myelopathy. J Neurol 250:1442–1452
Ford B, Tampieri D, Francis G (1992) Long-term follow-up of acute partial transverse myelopathy. Neurology 42:250–252
Wingerchuk DM, Lennon VA, Pittock SJ et al (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66(10):1485–1489
Weinshenker BG, Wingerchuk DM, Vukusic S et al (2006) Neuromyelitis optica IgG predicts relaps after longitudinally extensive transverse myelitis. Ann Neurol 59(3):566–569
Martiello M, Lennon VA, Jacob A et al (2008) NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology 70(23):2194–2200
Coulter I, Huda S, Baborie A et al (2012) Longitudinally extensive transverse myelitis as the sole presentation of neuro-Behcet’s disease responding to infliximab. J Spinal Cord Med 35(2):122–124
Eckstein C, Saidha S, Levy M (2012) A differential diagnosis of central nervous system demyelinisation: beyond multiple sclerosis. J Neurol 259:801–816
Haan J, Haupts M, Uhlenbrock D (1987) Magnetic resonance imaging (MRI), cranial computerized tomography (CCT), evoked potentials and cerebrospinal fluid (CSF) analysis in five patients with funicular myelosis. Neurosurg Rev 10(3):209–2011
Habek M, Adamec I, Pavlisa G et al (2012) Diagnostic approach of patients with longitudinally extensive transverse myelitis. Acta Neurol Belg 112(1):39–43
Kincaid O, Lipton HL (2006) Viral myelitis: an update. Curr Neurol Neurosci Rep 6(6):469–474
Rigamonti A, Usai S, Ciusani E et al (2005) Atypical transverse myelitis due to cytomegalovirus in a immunocompetent patient. Neurol Sci 26:351–354
Tyler KL, Gross RA, Cascino GD (1986) Unusual viral causes of transverse myelitis: hepatitis A virus and cytomegalovirus. Neurology 36:855–858
Yylmaz S, Koseolu HK, Yucel E (2007) Transverse myelitis caused by varicella zoster: case reports. Braz Infect Dis 11:179–181
Karunarathne S, Govindapala D, Udayakumara Y et al (2012) Cytomegalovirus associated transverse myelitis in an immunocompetent host with DNA detection in cerebrospinal fluid: a case report. BMC Res Notes 5(1):364 [Epub ahead of print]
Sanefuji M, Ohga S, Kira R et al (2008) Epstein-Barr virus-associated meningoencephalolmyelitis: intrathecal reactivation of the virus in an immunocompetent child. J Child Neurol 23(9):1072–1077
Bhat A, Naguwa S, Cheema G et al (2010) The epidemiology of transverse myelitis. Autoimmun Rev 9(5):A395–A399
Krishnan C, Kaplin AI, Graber JS et al (2005) Recurrent transverse myelitis following neurobrucellosis: immunologic features and beneficial response to immunosuppression. J Neurovirol 11(2):225–231
Shakir RA, Al-Din AS, Araj GF et al (1987) Clinical categories of neurobrucellosis. A report of 19 cases. Brain 110(1):213–223
Crook T, Bannister B (1996) Acute transverse myelitis associated with Chlamydia psittaci infection. J Infect 32(2):151–152
Bigi S, Aebi C, Nauer C et al (2010) Acute transverse myelitis in Lyme neuroborreliosis. Infection 38:413–416
Kayal AK, Goswami M, Das M et al (2011) Clinical spectrum of neurosyphilis in North East India. Neurol India 59(3):344–350
Garg RK, Sharma R, Kar AM, Kushawa RA et al (2010) Neurological complications of miliary tuberculosis. Clin Neurol Neurosurg 112(3):188–192
Alper G, Petropoulou P, Fitz CR et al (2011) Ideopathic acute transverse myelitis in children: an analysis and discussion of MRI findings. Mult Scler 17(1):74–80
Al Deeb SM, Yaqub BA, Bruyn GW et al (1997) Acute transverse myelitis—a localized form of post-infectious encephalomyelitis. Brain 120:1115–1122
Young NP, Weinshenker BG, Lucchinetti CF (2008) Acute disseminated encephalomyelitis: current understanding and controversies. Semin Neurol 28(1):84–94
Miller DH, Weinshenker BG, Filippi M et al (2008) Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 14(9):1157–1174
Jacob A, Weinshenker BG (2008) An approach to the diagnosis of acute transverse myelitis. Semin Neurol 28(1):105–120
Keegan M, Pittock S, Lennon V (2008) Autoimmune myelopathy associated with collapsin response-mediator protein-5 immunoglobulin G. Ann Neurol 63(4):531–534
Eiben RM, Dooley JP, Stowes SM et al (1965) Subacute necrotizing encephalopathy in infancy. Neurology 15:293 (Abstract)
Mizuguchi M (1997) Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev 19:81–92
Mastroyianni SD, Gionnis D, Voudris K et al (2006) Acute necrotizing encephylopathy of childhood in non-Asian patients: report of three cases and literature review. Child Neurol 21:872–879
Campistol J, Gassio R, Pineda M et al (1998) Acute necrotizing encephalopathy of childhood (infantile bilateral thalamic necrosis): two non-Japanese cases. Dev Med Child Neurol 40:771–774
Saji N, Yamamoto N, Yoda J et al (2006) Adult case of acute encephalopathy associated with bilateral thalamic lesions and peripheral neuropathy. No To Shinkei 58:1009–1014 (article in Japanese)
Miyata E (2002) An adult case of acute necrotizing encephalopathy. No To Shinkei 54:354–355 (article in Japanese)
Nakamura Y, Miura K, Yamada I et al (2000) A novel adult case of acute necrotizing encephalopathy of childhood with bilateral symmetric thalamic lesions. Rinsho Shinkeigaku 40:827–831 (article in Japanese)
Fasano A, Natoli GF, Cianfoni A et al (2007) Acute necrotizing encephalopathy. A relapsing case in an European adult. J Neuro Neurosurg Psychiatry 79(2):227–228
Kato H, Hasegawa H, Iijima M et al (2007) Brain magnetic resonance imaging of an adult case of acute necrotizing encephalopathy. J Neurol 254:1135–1137
Mizuguchi M, Yamanouchi H, Ichiyama T et al (2007) Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand 115:45–56
Mizoguchi M, Hayashi M, Nakano I et al (2002) Concentric structure of thalamic lesions in acute necrotizing encephalopathy. Neuroradiology 44:489–493
Kansagra SM, Gallentine WB (2011) Cytokine storm of acute necrotizing encephalopathy. Ped Neurol 45(6):400–402
Okumura A, Mizoguchi M, Kidokoro H et al (2009) Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev 31(3):221–227
Neilson DE, Eiben RM, Winiewski S et al (2003) Autosomal dominant acute necrotizing encephalopathy. Neurology 61:226–230
Neilson DE, Feiler HS, Wilhelmsen KC et al (2004) Autosomal dominant acute necrotizing encephalopathy maps to 2q12.1-2q. Ann Neurol 55:291–294
Bergamino L, Capra V, Biancheri R et al (2012) Immunomodulatory therapy in recurrent acute necrotizing encephalopathy ANE1: is it useful? Brain Dev 35(5):384–391
Cho KI, Cai Y, Yi H et al (2007) Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and funktion. Traffic 8:1722–1735
Aslanukov A, Bhowmick R, Guruju M et al (2006) RanBP2 modulates Cox11 and hexokinase I activities and haploinsufficiency of RanBP2 causes deficites in glucose metabolism. PLoS Genet 2(10):e177
Belay ED, Bresee JS, Holman RC et al (1999) Reye’s Syndrom in the United States from 1981 trough 1997. N Eng J Med 340(18):1377–1382
Neri M, Cantatore S, Pomara I et al (2011) Immunohistochemichal expression of proinflammatory cytokines Il-1beta, Il-6, TNF-alpha and involvement of COX-2, quantitatively confirmed by Western blot analysis. Wernicke’s encephalopathy. Pathol Res Pract 207(10):652–658
Brown GK, Squier MV (1996) Neuropathology and pathogenesis of mitochondrial diseases. J Inher Metab Dis 19(4):553–572
Acknowledgments
We thank the patient for her agreement into publishing her data and for her great support, especially in the establishment of her pedigree. The study was funded by the Swiss National Science Foundation and by the Clinical Research Priority Program Neuro-Rehab of the University of Zurich, Switzerland.
Conflicts of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolf, K., Schmitt-Mechelke, T., Kollias, S. et al. Acute necrotizing encephalopathy (ANE1): rare autosomal-dominant disorder presenting as acute transverse myelitis. J Neurol 260, 1545–1553 (2013). https://doi.org/10.1007/s00415-012-6825-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6825-7