Abstract
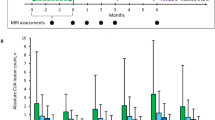
We herein provide a comprehensive assessment of magnetic resonance imaging (MRI) outcomes from CLARITY, a 96-week, double-blind study demonstrating significant clinical and MRI improvements in patients with relapsing–remitting multiple sclerosis (RRMS) treated with cladribine tablets. Patients with RRMS were randomized 1:1:1 to annual short-course therapy with cladribine tablets cumulative dose 3.5 or 5.25 mg/kg or placebo. MRI endpoints included mean number of T1 gadolinium-enhancing (Gd+), active T2 and combined unique (CU) lesions/patient/scan. MRI-measured disease activity was significantly reduced in both cladribine tablets groups versus placebo. The proportion of patients with no active lesions at study end was: T1 Gd+ lesions: 86.8 and 91.0 versus 48.3 % (p < 0.001); active T2 lesions: 61.7 and 62.5 versus 28.4 % (p < 0.001); CU lesions: 59.6 and 60.7 versus 26.1 % (p < 0.001). Clinically meaningful and significant reductions in active lesion counts and increases in proportions of active lesion-free patients were achieved consistently in cladribine tablet groups when data were stratified by baseline disease characteristics. For example, the percentage of patients who remained lesion-free over the study was significantly greater in cladribine tablet groups than in the placebo group for all lesion types regardless of relapse category at baseline (p < 0.001 for all analyses of patients with ≤1 or 2 relapses; p ≤ 0.022 for analyses of patients with ≥3 relapses). MRI-measured disease activity was greatly reduced by both doses of cladribine tablets, with consistent effect across clinically relevant patient populations. These findings add to our scientific understanding of the neurological impact of this therapeutic modality in patients with RRMS.




Similar content being viewed by others
References
Arnold DL (2007) The place of MRI in monitoring the individual MS patient. J Neurol Sci 259:123–127
Barkhof F, Polman CH, Radue EW, Kappos L, Freedman MS, Edan G, Hartung HP, Miller DH, Montalban X, Poppe P, de VM, Lasri F, Bauer L, Dahms S, Wagner K, Pohl C, Sandbrink R (2007) Magnetic resonance imaging effects of interferon beta-1b in the BENEFIT study: integrated 2-year results. Arch Neurol 64:1292–1298
Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 346:158–164
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Comi G (2010) Effects of disease modifying treatments on cognitive dysfunction in multiple sclerosis. Neurol Sci 31:S261–S264
Comi G, Pulizzi A, Rovaris M, Abramsky O, Arbizu T, Boiko A, Gold R, Havrdova E, Komoly S, Selmaj K, Sharrack B, Filippi M (2008) Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 371:2085–2092
Cook S, Vermersch P, Comi G, Giovannoni G, Rammohan K, Rieckmann P, Soelberg Sørensen P, Hamlett A, Miret M, Weiner J, Viglietta V, Musch B, Greenberg S (2011) Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (cladribine tablets treating multiple sclerosis orally) study. Mult Scler 17:578–593
Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, Thompson AJ, Miller DH (2008) Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 131:808–817
Gauthier SA, Mandel M, Guttmann CR, Glanz BI, Khoury SJ, Betensky RA, Weiner HL (2007) Predicting short-term disability in multiple sclerosis. Neurology 68:2059–2065
Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Sorensen PS, Vermersch P, Chang P, Hamlett A, Musch B, Greenberg SJ (2010) A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 362:416–426
Giovannoni G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, Vermersch P, Hamlett A, Viglietta V, Greenberg S (2011) Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post hoc and subgroup analysis. Lancet Neurol 10:329–337
Gold R, Kappos L, Bar-Or A, Arnold D, Giovannoni G, Selmaj K, Yang M, Dawson K, Bochum D, Lodz P (2011) Clinical efficacy of BG-12, an oral therapy, in relapsing-remitting multiple sclerosis: data from the phase 3 DEFINE trial. N Engl J Med 367:1098–1107
Goodman AD, Rossman H, Bar-Or A, Miller A, Miller DH, Schmierer K, Lublin F, Khan O, Bormann NM, Yang M, Panzara MA, Sandrock AW (2009) GLANCE: results of a phase 2, randomized, double-blind, placebo-controlled study. Neurology 72:806–812
Kappos L, Polman C, Pozzilli C, Thompson A, Beckmann K, Dahlke F (2001) Final analysis of the European multicenter trial on IFNbeta-1b in secondary-progressive MS. Neurology 57:1969–1975
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Kerbrat A, Le Page E, Leray E, Anani T, Coustans M, Desormeaux C, Guiziou C, Kassiotis P, Lallement F, Laplaud D, Diraison P, Rouhart F, Sartori E, Wardi R, Wiertlewski S, Edan G (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci 308:98–102
Leist TP, Vermersch P (2007) The potential role for cladribine in the treatment of multiple sclerosis: clinical experience and development of an oral tablet formulation. Curr Med Res Opin 23:2667–2676
Leist TP, Weissert R (2011) Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol 34:28–35
Lim SY, Constantinescu CS (2010) Current and future disease-modifying therapies in multiple sclerosis. Int J Clin Pract 64:637–650
Mikol DD, Barkhof F, Chang P, Coyle PK, Jeffery DR, Schwid SR, Stubinski B, Uitdehaag BM (2008) Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis [the REbif vs Glatiramer Acetate in Relapsing MS Disease (REGARD) study]: a multicentre, randomised, parallel, open-label trial. Lancet Neurol 7:903–914
Miller DH, Albert PS, Barkhof F, Francis G, Frank JA, Hodgkinson S, Lublin FD, Paty DW, Reingold SC, Simon J (1996) Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. US National MS Society Task Force. Ann Neurol 39:6–16
Miller DH, Soon D, Fernando KT, Macmanus DG, Barker GJ, Yousry TA, Fisher E, O’Connor PW, Phillips JT, Polman CH, Kappos L, Hutchinson M, Havrdova E, Lublin FD, Giovannoni G, Wajgt A, Rudick R, Lynn F, Panzara MA, Sandrock AW (2007) MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 68:1390–1401
Moraal B, Pohl C, Uitdehaag BM, Polman CH, Edan G, Freedman MS, Hartung HP, Kappos L, Miller DH, Montalban X, Lanius V, Sandbrink R, Barkhof F (2009) Magnetic resonance imaging predictors of conversion to multiple sclerosis in the BENEFIT study. Arch Neurol 66:1345–1352
Mostert JP, Koch MW, Steen C, Heersema DJ, De Groot JC, De Keyser J (2010) T2 lesions and rate of progression of disability in multiple sclerosis. Eur J Neurol 17:1471–1475
Nielsen JM, Pohl C, Polman CH, Barkhof F, Freedman MS, Edan G, Miller DH, Bauer L, Sandbrink R, Kappos L, Uitdehaag BM (2009) MRI characteristics are predictive for CDMS in monofocal, but not in multifocal patients with a clinically isolated syndrome. BMC Neurol 9:19
O’Riordan JI, Thompson AJ, Kingsley DP, Macmanus DG, Kendall BE, Rudge P, McDonald WI, Miller DH (1998) The prognostic value of brain MRI in clinically isolated syndromes of the CNS. A 10-year follow-up. Brain 121:495–503
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Prosperini L, Gallo V, Petsas N, Borriello G, Pozzilli C (2009) One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol 16:1202–1209
Rammohan K, Vermersch P, Comi G, Giovannoni G, Rieckmann P, Soelberg Sørensen P, Chang P, Hamlett A, Musch B, Verjee R, Greenberg S (2009) Cladribine tablets produce sustained improvements in relapsing-remitting multiple sclerosis (RRMS) in the 96-week, phase III, double-blind, placebo-controlled CLARITY study. Annual Congress of European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Dusseldorf, 9–12 September 2009 (abstract P818, p S249)
Rieckmann P, Comi G, Cook S, Giovannoni G, Rammohan K, Soelberg Sørensen P, Vermersch P, Chang P, Hamlett A, Viglietta V, Musch B, Greenberg S (2009) Effects of cladribine tablets on peripheral lymphocyte subtypes implicated in multiple sclerosis immunopathogenesis: surface marker analysis for a subset of patients from the 96 week, phase III, double-blind, placebo-controlled CLARITY study. Annual Congress of European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Dusseldorf, 9–12 September 2009 (abstract P816, p S248–S249)
Rudick RA, Lee JC, Simon J, Fisher E (2006) Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol 60:236–242
Ruggieri M, Avolio C, Livrea P, Trojano M (2007) Glatiramer acetate in multiple sclerosis: a review. CNS Drug Rev 13:178–191
Scott TF, Schramke CJ, Novero J, Chieffe C (2000) Short-term prognosis in early relapsing-remitting multiple sclerosis. Neurology 55:689–693
Simon JH, Li D, Traboulsee A, Coyle PK, Arnold DL, Barkhof F, Frank JA, Grossman R, Paty DW, Radue EW, Wolinsky JS (2006) Standardized MR imaging protocol for multiple sclerosis: consortium of MS centers consensus guidelines. Am J Neuroradiol 27:455–461
Soelberg Sørensen P, Comi G, Cook S, Giovannoni G, Rammohan K, Rieckmann P, Vermersch P, Chang P, Hamlett A, Viglietta V, Verjee R, Musch B, Greenberg S (2009) Effects of cladribine tablets on haematological profiles in patients with relapsing-remitting multiple sclerosis (RRMS) in the 96 week, phase III, double-blind, placebo-controlled CLARITY study. Annual Congress of European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Dusseldorf, 9–12 September 2009 (abstract P472, p S137)
Stadelmann C (2011) Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol 24:224–229
Tintore M (2009) New options for early treatment of multiple sclerosis. J Neurol Sci 277:S9–S11
Trojano M, Pellegrini F, Fuiani A, Paolicelli D, Zipoli V, Zimatore GB, Di Monte E, Portaccio E, Lepore V, Livrea P, Amato MP (2007) New natural history of interferon-beta-treated relapsing multiple sclerosis. Ann Neurol 61:300–306
Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Zimatore GB, Tortorella C, Simone IL, Patti F, Ghezzi A, Portaccio E, Rossi P, Pozzilli C, Salemi G, Lugaresi A, Bergamaschi R, Millefiorini E, Clerico M, Lus G, Vianello M, Avolio C, Cavalla P, Iaffaldano P, Direnzo V, D’Onghia M, Lepore V, Livrea P, Comi G, Amato MP (2009) Post-marketing of disease modifying drugs in multiple sclerosis: an exploratory analysis of gender effect in interferon beta treatment. J Neurol Sci 286:109–113
Vermersch P, Comi G, Cook S, Giovannoni G, Rammohan K, Rieckmann P, Soelberg Sørensen P, Chang P, Hamlett A, Musch B, Verjee R, Greenberg S (2009) Early onset of effect of treatment with cladribine tablets for relapsing-remitting multiple sclerosis in the 96-week, phase III, double-blind, placebo-controlled CLARITY study. Annual Congress of European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Dusseldorf, 9–12 September 2009 (abstract P817, p S249)
Acknowledgments
This study was sponsored by Merck Serono S.A., Geneva, Switzerland, a branch of Merck Serono S.A. Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. The authors thank Peter Chang (formerly of Merck Serono S.A., Geneva, Switzerland) for his input into the statistical analysis of data for this manuscript, and Mary Goodsell of ACUMED®, Tytherington, UK (supported by Merck Serono, S.A., Geneva, Switzerland) for her assistance in the preparation of this manuscript.
Conflicts of interest
Professor Comi has received consulting fees for participating on advisory boards in relation to clinical trial design, trial steering committees and data and safety monitoring committees from Novartis, Teva Pharmaceutical Ind. Ltd., Sanofi-Aventis, Merck Serono, and Bayer Schering; lecture fees from Novartis, Teva Pharmaceutical Ind. Ltd., Sanofi-Aventis, Merck Serono, Biogen Dompè, Bayer Schering and Serono Symposia International Foundation; and clinical trial grant support from Novartis, Teva Pharmaceutical Ind. Ltd., Sanofi-Aventis, Merck Serono, Biogen Dompè, and Bayer Schering. Professor Cook has received honoraria for lectures from Merck Serono and Bayer; and consultation fees from Sanofi-Aventis, Bayer, Biogen Idec, Merck Serono, Actinobac Scientific, Inc., and Teva. He has served on scientific advisory boards for Bayer, Biogen Idec, Merck Serono and Actinobac Scientific, Inc. He has received research grants for the BEYOND and BECOME studies from Bayer and the CLARITY and CLARITY EXTENSION studies from Merck Serono. Professor Giovannoni has received consulting fees for participating on advisory boards in relation to clinical trial design, trial steering committees and data and safety monitoring committees from Bayer-Schering, Biogen Idec, Five Prime Therapeutics, Genzyme, Ironwood Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Aventis, Teva-Aventis, UCB Pharma, and Vertex Pharmaceuticals; lecture fees from Bayer-Schering, Biogen Idec, Genzyme, Merck Serono, and Vertex Pharmaceuticals; and grant support from Bayer-Schering, Biogen Idec, GW Pharmaceuticals, Ironwood Pharmaceuticals, Merck Serono, Merz, Novartis, and Teva-Aventis. Professor Rammohan has received consulting fees for speaker and consultancy activities from Bayer, EMD Serono/Pfizer, Teva, Genentech, Biogen, Genzyme, Acorda, UCB Pharma, and Novartis; lecture fees from Bayer, EMD Serono/Pfizer, Teva, Biogen, Genzyme, Acorda, and UCB Pharma; and clinical trial and investigator initiated research grant support from Bayer, EMD Serono/Pfizer, Teva, Acorda, Biogen, Genzyme, UCB Pharma, and Novartis. Professor Rieckmann has received consulting fees for speaker and consultancy activities from Merck Serono, Biogen Idec, Teva, Bayer, and Novartis; lecture fees from Merck Serono, Biogen Idec, and Teva; and grant support from Merck Serono, Bayer, Teva, the MS Society of Canada, and the Ilich Foundation. Professor Soelberg Sørensen has served on scientific advisory boards for Biogen Idec, Merck Serono, Novartis, Genmab, Teva, Elan, GSK, steering committees or independent data monitoring boards in clinical trials sponsored by Merck Serono, Genmab, Teva, GSK, Bayer-Schering, and received funding of travel for these activities. He has served as Editor-in-Chief of the European Journal of Neurology, and is editorial board member for Therapeutic Advances in Neurological Disorders and Multiple Sclerosis; has received speaker honoraria from Biogen Idec, Merck Serono, Teva, Bayer Schering, Sanofi-Aventis, and Novartis; and has received research support from Biogen Idec, Bayer-Schering, Merck Serono, Teva, Baxter, Sanofi-Aventis, BioMS, Novartis, Bayer, RoFAR, Roche, Genzyme, from the Danish Multiple Sclerosis Society, the Danish Medical Research Council, and the European Union Sixth Framework Programme: Life sciences, Genomics and Biotechnology for Health. Professor Vermersch has received consulting fees for speaker and advisory board activities from Merck Serono, Bayer-Schering, Teva-Aventis, Biogen Idec, Novartis, and Roche; lecture fees from Merck Serono, Biogen Idec, Bayer-Schering, and Novartis; and grant support from Biogen Idec, Bayer-Schering Pharma, Merck Serono, and Teva-Aventis. Dr. Hamlett, Dr. Greenberg and Dr. Viglietta are current or former employees of the study sponsor (Merck Serono S.A., Geneva, Switzerland, a branch of Merck Serono S.A. Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany). Dr. Greenberg is currently an employee of Abbott Biotherapeutics Corp, and Dr. Viglietta is currently an employee of Biogen Idec.
Ethical standards
The protocol was reviewed and approved by the local review board or ethics committee at each study center. An independent data and safety monitoring board reviewed the study conduct and all safety data.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the CLARITY study group.
V. Viglietta and S. J. Greenberg were in Merck Serono S.A., Geneva, Switzerland at the time of manuscript preparation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Comi, G., Cook, S.D., Giovannoni, G. et al. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol 260, 1136–1146 (2013). https://doi.org/10.1007/s00415-012-6775-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6775-0