Abstract
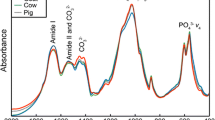
In forensic anthropology, the application of traditional methods for estimating the biological profile of human skeletal remains is often hampered by poor preservation and skeletal representativeness, compromising their reliability. Thus, the development of alternative methods to the morphometric analysis of bones to estimate the biological profile of human remains is paramount. The age of an individual can cause changes in bone morphology, mass and size, as well as in its chemical composition. In this sense, the main objective of this research was to evaluate if the contents of bone collagen (Am/P), carbonate type A (API), carbonate type B (BPI), the relation between the carbonate content (types A and B) to type B carbonate (C/C), carbonate-phosphate ratio (C/P) and crystallinity index (CI), spectroscopic indices obtained from relationships between infrared absorption band intensities (FTIR-ATR), can be used as age-at-death predictors. A sample of femora and humeri from the 21st Century Identified Skeleton Collection (N = 80, 44 females and 36 males) was employed. Results show that, with advancing age, women’s femora have lower CI values, but BPI and C/P indices increase, and the deformation and disorder of the crystal lattice are probably affected by the integration of type B carbonate content of the femur. The ratios analysed, especially the CI and the BPI, show potential to estimate age-at-death in human skeletal remains, when sex is already known, thus helping to assess the biological profile when conventional methods cannot be applied.

Similar content being viewed by others
References
Cattaneo C (2007) Forensic anthropology: developments of a classical discipline in the new millennium. Forensic Sci Int 165:185–193. https://doi.org/10.1016/j.forsciint.2006.05.018
Cunha E (2014) A Antropologia Passo a Passo. In: Gomes A (ed) Enfermagem Forense. Lidel, Lisboa, pp 280–288
Mundorff AZ, Shaler R, Bieschke ET, Mar-Cash E (2014) Marrying anthropology and DNA: essential for solving complex commingling problems in cases of extreme fragmentation. In: Adams BJ, Byrd JE (eds) Recovery, analysis, and identification of commingled human remains. Academic Press, San Diego, pp 257–273
Yazedjian L, Kešetović R (2008) The application of traditional anthropological methods in a DNA-led identification process. In: Adams BJ, Byrd JE (eds) recovery, analysis, and identification of commingled human remains. Humana Press, pp 271–284
Boskey JA, Gokhale AL, Robey PG (2001) The biochemistry of bone. In: Marcus R, Feldman D, Nelson D, Rosen C (eds) Osteoporosis I. Academic Press, San Diego, pp 107–188
Wang X-Y, Zuo Y, Huang D et al (2010) Comparative study on inorganic composition and crystallographic properties of cortical and cancellous bone. Biomed Environ Sci 23:473–480. https://doi.org/10.1016/S0895-3988(11)60010-X
Boskey AL, Robey PG (2007) The composition of bone. In: Bilezikian JP, Bouillon R, Clemens T et al (eds) Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Wiley-Blackwell, pp 84–92
Zhu W, Robey PG, Boskey AL (2013) The regulatory role of matrix proteins in mineralization of bone. In: Marcus R, Feldman D, Nelson D, Rosen C (eds) Osteoporosis I. Academic Press, San Diego, pp 235–255
Gonçalves D, Vassalo AR, Mamede AP, Makhoul C, Piga G, Cunha E, Marques MPM, Batista de Carvalho LAE (2018) Crystal clear: vibrational spectroscopy reveals intrabone, intraskeleton, and interskeleton variation in human bones. Am J Phys Anthropol 166:296–312. https://doi.org/10.1002/ajpa.23430
Nagy G, Lorand T, Patonai Z, Montsko G, Bajnoczky I, Marcsik A, Mark L (2008) Analysis of pathological and non-pathological human skeletal remains by FT-IR spectroscopy. Forensic Sci Int 175:55–60. https://doi.org/10.1016/j.forsciint.2007.05.008
Marques MPM, Gonçalves D, Amarante AIC, Makhoul CI, Parker SF, Batista de Carvalho LAE (2016) Osteometrics in burned human skeletal remains by neutron and optical vibrational spectroscopy. RSC Adv 6:68638–68641. https://doi.org/10.1039/c6ra13564a
Paschalis EP, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL (1996) FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int 59:480–487. https://doi.org/10.1007/BF00369214
Madupalli H, Pavan B, Tecklenburg MMJ (2017) Carbonate substitution in the mineral component of bone: discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J Solid State Chem 255:27–35. https://doi.org/10.1016/j.jssc.2017.07.025
Mamede AP, Gonçalves D, Marques MPM, Batista de Carvalho LAE (2018) Burned bones tell their own stories: a review of methodological approaches to assess heat-induced diagenesis. Appl Spectrosc Rev 53:603–635. https://doi.org/10.1080/05704928.2017.1400442
Tacker RC (2008) Carbonate in igneous and metamorphic fluorapatite: two type A and two type B substitutions. Am Mineral 93:168–176. https://doi.org/10.2138/am.2008.2551
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25:131–143. https://doi.org/10.1016/j.msec.2005.01.008
Carden A, Morris MD (2000) Application of vibrational spectroscopy to the study of mineralized tissues (review). J Biomed Opt 5:259–268. https://doi.org/10.1117/1.429994
Person A, Bocherens H, Saliège JF, Paris F, Zeitoun V, Gérard M (1995) Early diagenetic evolution of bone phosphate: an X-ray diffractometry analysis. J Archaeol Sci 22:211–221. https://doi.org/10.1006/jasc.1995.0023
Boskey A, Pleshko Camacho N (2007) FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 28:2465–2478. https://doi.org/10.1016/j.biomaterials.2006.11.043
Lebon M, Reiche I, Bahain JJ, Chadefaux C, Moigne AM, Fröhlich F, Sémah F, Schwarcz HP, Falguères C (2010) New parameters for the characterization of diagenetic alterations and heat-induced changes of fossil bone mineral using Fourier transform infrared spectrometry. J Archaeol Sci 37:2265–2276. https://doi.org/10.1016/j.jas.2010.03.024
Marques MPM, Mamede AP, Vassalo AR, Makhoul C, Cunha E, Gonçalves D, Parker SF, Batista de Carvalho LAE (2018) Heat-induced bone diagenesis probed by vibrational spectroscopy. Sci Rep 8:15935. https://doi.org/10.1038/s41598-018-34376-w
Mamede AP, Vassalo AR, Piga G, Cunha E, Parker SF, Marques MPM, Batista de Carvalho LAE, Gonçalves D (2018) Potential of bioapatite hydroxyls for research on archeological burned bone. Anal Chem 90:11556–11563. https://doi.org/10.1021/acs.analchem.8b02868
Vassalo AR, Cunha E, Batista de Carvalho LAE, Gonçalves D (2016) Rather yield than break: assessing the influence of human bone collagen content on heat-induced warping through vibrational spectroscopy. Int J Legal Med 130:1647–1656. https://doi.org/10.1007/s00414-016-1400-x
Beasley MM, Bartelink EJ, Taylor L, Miller RM (2014) Comparison of transmission FTIR, ATR, and DRIFT spectra: implications for assessment of bone bioapatite diagenesis. J Archaeol Sci 46:16–22. https://doi.org/10.1016/j.jas.2014.03.008
Dutta A (2017) Fourier transform infrared spectroscopy. In: Thomas S, Thomas R, Zachariah AK, Mishra RK (eds) Spectroscopic Methods for Nanomaterials Characterization. Elsevier Inc, pp 73–93
Thompson TJU, Gauthier M, Islam M (2009) The application of a new method of Fourier transform infrared spectroscopy to the analysis of burned bone. J Archaeol Sci 36:910–914. https://doi.org/10.1016/j.jas.2008.11.013
Monnier GF (2018) A review of infrared spectroscopy in microarchaeology: methods, applications, and recent trends. J Archaeol Sci Rep 18:806–823. https://doi.org/10.1016/j.jasrep.2017.12.029
Stiner MC, Kuhn SL, Surovell TA, Goldberg P, Meignen L, Weiner S, Bar-Yosef O (2001) Bone preservation in Hayonim Cave (Israel): a macroscopic and mineralogical study. J Archaeol Sci 28:643–659. https://doi.org/10.1006/jasc.2000.0634
Hollund HI, Ariese F, Fernandes R et al (2013) Testing an alternative high-throughput tool for investigating bone diagenesis: FTIR in attenuated total reflection (ATR) mode. Archaeometry 55:507–532. https://doi.org/10.1111/j.1475-4754.2012.00695.x
Legros R, Balmain N, Bonel G (1987) Age-related changes in mineral of rat and bovine cortical bone. Calcif Tissue Int 41:137–144. https://doi.org/10.1007/BF02563793
Rey C, Renugopalakrishman V, Collins B, Glimcher MJ (1991) Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif Tissue Int 49:251–258. https://doi.org/10.1007/BF02556214
Handschin RG, Stern WB (1995) X-ray diffraction studies on the lattice perfection of human bone apatite (Crista Iliaca). Bone 16:355S–363S. https://doi.org/10.1016/S8756-3282(95)80385-8
Miller LM, Vairavamurthy V, Chance MR, Mendelsohn R, Paschalis EP, Betts F, Boskey AL (2001) In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the ν4 PO43− vibration. Biochim Biophys Acta, Gen Subj 1527:11–19. https://doi.org/10.1016/S0304-4165(01)00093-9
Grynpas MD, Bonar LC, Glimcher MJ (1984) Failure to detect an amorphous calcium-phosphate solid phase in bone mineral: a radial distribution function study. Calcif Tissue Int 36:291–301. https://doi.org/10.1007/BF02405333
Handschin RG, Stern WB (1992) Crystallographic lattice refinement of human bone. Calcif Tissue Int 51:111–120. https://doi.org/10.1007/BF00298498
Rey C, Kim HM, Glimcher MJ (1994) Maturation of poorly crystalline synthetic and biological apatites. In: Brown PW, Constantz B (eds) Hydroxyapatite and related materials. CRC Press, Boca Raton, pp 181–187
Gourion-Arsiquaud S, Burket JC, Havill LM, DiCarlo E, Doty SB, Mendelsohn R, van der Meulen MCH, Boskey AL (2009) Spatial variation in osteonal bone properties relative to tissue and animal age. J Bone Miner Res 24:1271–1281. https://doi.org/10.1359/jbmr.090201
Turunen MJ, Saarakkala S, Rieppo L, Helminen HJ, Jurvelin JS, Isaksson H (2011) Comparison between infrared and Raman spectroscopic analysis of maturing rabbit cortical bone. Appl Spectrosc 65:595–603. https://doi.org/10.1366/10-06193
Sillen A, Morris A (1996) Diagenesis of bone from border cave: implications for the age of the border cave hominids. J Hum Evol 31:499–506. https://doi.org/10.1006/jhev.1996.0075
Grynpas MD, Tupy JH, Sodek J (1994) The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone 15:505–513. https://doi.org/10.1016/8756-3282(94)90274-7
Fedarko NS, Vetter UK, Weinstein S, Robey PG (1992) Age-related changes in hyaluronan, proteoglycan, collagen, and osteonectin synthesis by human bone cells. J Cell Physiol 151:215–227. https://doi.org/10.1002/jcp.1041510202
Ferreira MT, Vicente R, Navega D et al (2014) A new forensic collection housed at the University of Coimbra, Portugal: the 21st century identified skeletal collection. Forensic Sci Int 245:202.e1–202.e5. https://doi.org/10.1016/j.forsciint.2014.09.021
Boaks A, Siwek D, Mortazavi F (2014) The temporal degradation of bone collagen: a histochemical approach. Forensic Sci Int 240:104–110. https://doi.org/10.1016/j.forsciint.2014.04.008
Grupe G (1988) Impact of the choice of bone samples on trace element data in excavated human skeletons. J Archaeol Sci 15:123–129. https://doi.org/10.1016/0305-4403(88)90002-7
Weiner S, Bar-Yosef O (1990) States of preservation of bones from prehistoric sites in the near east: a survey. J Archaeol Sci 17:187–196. https://doi.org/10.1016/0305-4403(90)90058-D
Wright LE, Schwarcz HP (1996) Infrared and isotopic evidence for diagenesis of bone apatite at Dos Pilas, Guatemala: palaeodietary implications. J Archaeol Sci 23:933–944. https://doi.org/10.1006/jasc.1996.0087
Trueman CNG, Behrensmeyer AK, Tuross N, Weiner S (2004) Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenya: diagenetic mechanisms and the role of sediment pore fluids. J Archaeol Sci 31:721–739. https://doi.org/10.1016/j.jas.2003.11.003
Snoeck C, Lee-Thorp JA, Schulting RJ (2014) From bone to ash: compositional and structural changes in burned modern and archaeological bone. Palaeogeogr Palaeoclimatol Palaeoecol 416:55–68. https://doi.org/10.1016/j.palaeo.2014.08.002
Team RC (2019) R: A language and environment for statistical computing. http://r-project.org/. Accessed 4 Nov 2019
Reznikov N, Chase H, Brumfeld V, Shahar R, Weiner S (2015) The 3D structure of the collagen fibril network in human trabecular bone: relation to trabecular organization. Bone 71:189–195. https://doi.org/10.1016/j.bone.2014.10.017
Thompson TJU, Islam M, Bonniere M (2013) A new statistical approach for determining the crystallinity of heat-altered bone mineral from FTIR spectra. J Archaeol Sci 40:416–422. https://doi.org/10.1016/j.jas.2012.07.008
Francalacci P, Tarli SB (1988) Multielementary analysis of trace elements and preliminary results on stable isotopes in two Italian prehistoric sites. Methodological aspects. In: Grupe G, Herrmann B (eds) Trace elements in environmental history. Proceedings in Life Sciences. Springer, Berlin, pp 41–52
Shemesh A (1990) Crystallinity and diagenesis of sedimentary apatites. Geochim Cosmochim Acta 54:2433–2438. https://doi.org/10.1016/0016-7037(90)90230-I
Stiner MC, Kuhn SL, Weiner S, Bar-Yosef O (1995) Differential burning, recrystallization, and fragmentation of archaeological bone. J Archaeol Sci 22:223–237. https://doi.org/10.1006/jasc.1995.0024
Trueman CN, Privat K, Field J (2008) Why do crystallinity values fail to predict the extent of diagenetic alteration of bone mineral? Palaeogeogr Palaeoclimatol Palaeoecol 266:160–167. https://doi.org/10.1016/j.palaeo.2008.03.038
Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R (2005) Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int 16:2031–2038. https://doi.org/10.1007/s00198-005-1992-3
Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB (2003) Aging of microstructural compartments in human compact bone. J Bone Miner Res 18:1012–1019. https://doi.org/10.1359/jbmr.2003.18.6.1012
Thompson TJU, Islam M, Piduru K, Marcel A (2011) An investigation into the internal and external variables acting on crystallinity index using Fourier transform infrared spectroscopy on unaltered and burned bone. Palaeogeogr Palaeoclimatol Palaeoecol 299:168–174. https://doi.org/10.1016/j.palaeo.2010.10.044
Driessens FCM, van Dijk JWE, Borggreven JMPM (1978) Biological calcium phosphates and their role in the physiology of bone and dental tissues I. composition and solubility of calcium phosphates. Calcif Tissue Res 26:127–137. https://doi.org/10.1007/BF02013247
Glimcher MJ (2006) Bone: nature of the calcium phosphate crystals and cellular, structural, and physical chemical mechanisms in their formation. Rev Mineral Geochem 64:223–282. https://doi.org/10.2138/rmg.2006.64.8
Surovell TA, Stiner MC (2001) Standardizing infra-red measures of bone mineral crystallinity: an experimental approach. J Archaeol Sci 28:633–642. https://doi.org/10.1006/jasc.2000.0633
Rey C, Collins B, Goehl T, Dickson IR, Glimcher MJ (1989) The carbonate environment in bone mineral: a resolution-enhanced Fourier transform infrared spectroscopy study. Calcif Tissue Int 45:157–164. https://doi.org/10.1007/BF02556059
Pleshko N, Boskey A, Mendelsohn R (1991) Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys J 60:786–793. https://doi.org/10.1016/S0006-3495(91)82113-0
Figueiredo MM, Gamelas JAF, Martins AG (2012) Characterization of bone and bone-based graft materials using FTIR spectroscopy. In: Theophanides T (ed) Infrared spectroscopy. IntechOpen, Rijeka, pp 315–338
Wang Q, Li W, Liu R, Zhang K, Zhang H, Fan S, Wang Z (2019) Human and non-human bone identification using FTIR spectroscopy. Int J Legal Med 133:269–276. https://doi.org/10.1007/s00414-018-1822-8
Nielsen-Marsh CM, Hedges REM (2000) Patterns of diagenesis in bone II: effects of acetic acid treatment and the removal of diagenetic CO32−. J Archaeol Sci 27:1151–1159. https://doi.org/10.1006/jasc.1999.0538
Longato S, Wöss C, Hatzer-Grubwieser P, Bauer C, Parson W, Unterberger SH, Kuhn V, Pemberger N, Pallua AK, Recheis W, Lackner R, Stalder R, Pallua JD (2015) Post-mortem interval estimation of human skeletal remains by micro-computed tomography, mid-infrared microscopic imaging and energy dispersive X-ray mapping. Anal Methods 7:2917–2927. https://doi.org/10.1039/c4ay02943g
Akkus O, Adar F, Schaffler MB (2004) Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone 34:443–453. https://doi.org/10.1016/j.bone.2003.11.003
Yerramshetty JS, Lind C, Akkus O (2006) The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone 39:1236–1243. https://doi.org/10.1016/j.bone.2006.06.002
Klepinger LL (2006) Fundamentals of forensic anthropology. John Wiley & Sons, Franklin Township
Rey C, Combes C, Drouet C, Sfihi H, Barroug A (2007) Physico-chemical properties of nanocrystalline apatites: implications for biominerals and biomaterials. Mater Sci Eng C 27:198–205. https://doi.org/10.1016/j.msec.2006.05.015
Pucéat E, Reynard B, Lécuyer C (2004) Can crystallinity be used to determine the degree of chemical alteration of biogenic apatites? Chem Geol 205:83–97. https://doi.org/10.1016/j.chemgeo.2003.12.014
Casuccio C (1962) An introduction to the study of osteoporosis (biochemical and biophysical research in bone ageing). Proc R Soc Med 55:663–668. https://doi.org/10.1177/003591576205500815
Rogers HJ, Weidmann SM, Parkinson A (1952) Studies on the skeletal tissues. II. The collagen content of bones from rabbits, oxen and humans. Biochem J 50:537–542. https://doi.org/10.1042/bj0500537
Eastoe JE (1968) Chemical aspects of the matrix concept in calcified tissue organisation. Calcif Tissue Res 2:1–19. https://doi.org/10.1007/BF02279189
Very JM, Gibert R, Guilhot B, Debout M, Alexandre C (1997) Effect of aging on the amide group of bone matrix, measured by FTIR spectrophotometry, in adult subjects deceased as a result of violent death. Calcif Tissue Int 60:271–275. https://doi.org/10.1007/s002239900228
Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L (1999) Age-related changes in the biochemical properties of human cancellous bone collagen: relationship to bone strength. Calcif Tissue Int 65:203–210. https://doi.org/10.1007/s002239900683
Zioupos P, Currey JD, Hamer AJ (1999) The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res 45:108–116. https://doi.org/10.1002/(SICI)1097-4636(199905)45:2<108::AID-JBM5>3.0.CO;2-A
Collins MJ, Nielsen-Marsh CM, Hiller J, Smith CI, Roberts JP, Prigodich RV, Wess TJ, Csapo J, Millard AR, Turner-Walker G (2002) The survival of organic matter in bone: a review. Archaeometry 44:383–394. https://doi.org/10.1111/1475-4754.t01-1-00071
Ruppel ME, Burr DB, Miller LM (2006) Chemical makeup of microdamaged bone differs from undamaged bone. Bone 39:318–324. https://doi.org/10.1016/J.BONE.2006.02.052
Dequeker J, Merlevede W (1971) Collagen content and collagen extractability pattern of adult human trabecular bone according to age, sex and amount of bone mass. Biochim Biophys Acta, Gen Subj 244:410–420. https://doi.org/10.1016/0304-4165(71)90243-1
Dequeker J, Remans J, Franssen R, Waes J (1971) Ageing patterns of trabecular and cortical bone and their relationship. Calcif Tissue Res 7:23–30. https://doi.org/10.1007/BF02062590
Baccino E, Schmitt A (2006) Determination of adult age at death in the forensic context. In: Schmitt A, Cunha E, Pinheiro J (eds) Forensic anthropology and medicine: complementary sciences from recovery to cause of death. Humana Press, Totowa, pp 259–280
Funding
The authors thank the Centre for Functional Ecology, the Research Centre for Anthropology and Health and the Molecular Physical Chemistry R&D Unit (financed by national funds by FCT – Fundação para a Ciência e Tecnologia, under the projects UID/BIA/04004/2019, UID/SADG/00283/2019 and UIDB/00070/2020, respectively).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pedrosa, M., Curate, F., Batista de Carvalho, L.A.E. et al. Beyond metrics and morphology: the potential of FTIR-ATR and chemometrics to estimate age-at-death in human bone. Int J Legal Med 134, 1905–1914 (2020). https://doi.org/10.1007/s00414-020-02310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-020-02310-3