Abstract
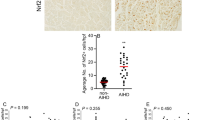
Although establishing agony is crucial in forensic practice, the identification of specific signs indicative of a detailed duration of agony is however not of immediate execution. Nitric oxide (NO) is the most important messenger molecule in the modulation of vascular tone and it is produced during stress conditions by inducible nitric oxide synthase (iNOS), as occurs during agony. The aim of this study was to investigate the relationship between immunohistochemical expression of iNOS, and agonal time (T), defined as the interval between the onset of a hypoxic-ischemic injury and the death. INOS expression was evaluated by measuring the average of signal intensity (SI) from cytoplasm of 300 smooth muscle cells of sample of renal artery, performed by ImageJ software: high values of SI correspond to a low enzyme expression and vice versa. We aimed also to check if gender, age, type of death (violent or natural death), post mortem interval, and storage in cold chamber influenced SI. We assessed 50 autopsied cases, of which 28 violent and 22 natural deaths, with a well-known T in a range between 1 and 631 min. Statistical analysis was performed to estimate the relationship between SI and the other variables. Results pointed out that only SI is related to T, and since data showed a bi-phase relationship between T and SI, we used a piecewise regression method for estimation of T as function of SI. The transition from the first to the second phase takes place at SI = 117.5 which corresponds to a T of 29.5 min. In conclusion, the study demonstrates that iNOS is a good marker for estimating T and the final regression model can be used in many forensic activities.




Similar content being viewed by others
References
Puccini C (2007) Istituzioni di Medicina Legale. CEA, Milano
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43(2):109–142
Förstermann U, Schmidt HH, Pollock JS, Sheng H, Mitchell JA, Warner TD, Nakane M, Murad F (1991) Isoforms of nitric oxide synthase: characterization and purincation from different cell types. Biochem Pharmacol 42:1849–1857
Villanueva C, Giulivi C (2010) Subcellular and cellular locations of nitric-oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med 49(3):307–316
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837
Wong JM, Billiar TR (1995) Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv Pharmacol 34:155–170
Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AH, Billiar TR, Tweardy DJ (1998) Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med 187(6):917–928
Umbrello M, Dyson A, Feelisch M, Singer M (2013) The key role of nitric oxide in hypoxia: hypoxic vasodilation and energy supply–demand matching. Antioxid Redox Signal 19(14):1690–1710
Bogdan C (2011) Regulation of lymphocytes by nitric oxide. Methods Mol Biol 677:375–393
Joles JA, Vos IH, Grone HJ et al (2002) Inducible nitric oxide synthase in renal transplantation. Kidney Int 61:872–875
Legrand M, Mik EG, Johannes T, Payen D, Ince C (2008) Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med 14(7–8):502–516
Ohishi M, Dusting GJ, Fennessy PA, Mendelsohn FA, Li XC, Zhuo JL (2010) Increased expression and co-localization of ACE, angiotensin II AT receptors and inducible nitric oxide synthase in atherosclerotic human coronary arteries. Int J Physiol Pathophysiol Pharmacol 2(2):111–124
Rasband WS (1997–2014) ImageJ. U. S. National Institutes of Health, Bethesda. http://imagej.nih.gov/ij/
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, URL http://www.R-project.org/
Muggeo VMR (2003) Estimating regression models with unknown break-points. Stat Med 22:3055–3071
Ikematsu K, Tsuda R, Kondo T, Kondo H, Ozawa K, Ogawa S, Nakasono I (2004) The expression of ‘150-kDa oxygen regulated protein (ORP-150)’ in human brain and its relationship with duration time until death. Legal Med 6(2):97–101
Piette MH, Pieters SE, De Letter EA (2011) Evaluation of the agonal stress: can immunohistochemical detection of ubiquitin in the locus coeruleus be useful? Int J Legal Med 125(3):333–340
Ito S, Abe K (1997) Contractile properties of afferent and efferent arterioles. Clin Exp Pharmacol Physiol 24:532–535
Pallone TL, Silldorff EP (2001) Pericyte regulation of renal medullary blood flow. Exp Nephrol 9:165–170
Leong CL, Anderson WP, O’Connor PM, Evans RG (2007) Evidence that renal arterial-venous oxygen shunting contributes to dynamic regulation of renal oxygenation. Am J Physiol Ren Physiol 292:1726–1733
Akaike T, Maeda H (1996) Quantitation of nitric oxide using 2-phenyl-4,4,5,5-tetramethylimidazoline-l-oxyl-3-oxide (PTIO). Methods Enzymol 268:211–221
Orihara Y, Ikematsu K, Tsuda R, Nakasono I (2001) Induction of nitric oxide synthase by traumatic brain injury. Forensic Sci Int 123:142–149
Gahm C, Holmin S, Mathiesen T (2002) Nitric oxide synthase expression after human brain contusion. Neurosurgery 50(6):1319–1326
Guzik TJ, Korbut R, Adamek-Guzik TJ (2003) Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol 54(4):469–487
Acknowledgments
This work was supported by grants from Polytechnic University of Marches. We will like to thank all members of Section of Legal Medicine of Polytechnic University of Marches for providing the samples of tissues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our investigations were conducted in accordance with the human and ethical principles of our university.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Scendoni, R., Ferrante, L., Stramazzotti, D. et al. Analysis of immunohistochemical expression of inducible nitric oxide synthase for the evaluation of agonal time in forensic medicine. Int J Legal Med 130, 1639–1646 (2016). https://doi.org/10.1007/s00414-016-1402-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-016-1402-8