Abstract
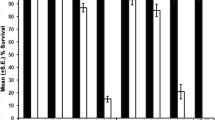
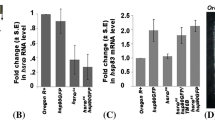
The hs-GAL4 t-driven expression of the hsrω-RNAi transgene or EP93D allele of the noncoding hsrω resulted in global down- or upregulation, respectively, of the large hsrω-n transcripts following heat shock. Subsequent to temperature shock, hsrω-null or those expressing hsrω-RNAi or the EP93D allele displayed delayed lethality of most embryos, first or third instar larvae. Three-day-old hsrω-null flies mostly died immediately or within a day after heat shock. Heat-shock-induced RNAi or EP expression in flies caused only a marginal lethality but severely affected oogenesis. EP allele or hsrω-RNAi expression after heat shock did not affect heat shock puffs and Hsp70 synthesis. Both down- and upregulation of hsrω-n transcripts suppressed reappearance of the hsrω-n transcript-dependent nucleoplasmic omega speckles during recovery from heat shock. Hrp36, heterochromatin protein 1, and active RNA pol II in unstressed or heat-shocked wild-type or hsrω-null larvae or those expressing the hs-GAL4 t-driven hsrω-RNAi or the EP93D allele were comparably distributed on polytene chromosomes. Redistribution of these proteins to pre-stress locations after a 1- or 2-h recovery was severely compromised in glands with down- or upregulated levels of hsrω-n transcripts after heat shock. The hsrω-null unstressed cells always lacked omega speckles and little Hrp36 moved to any chromosome region following heat shock, and its relocation to chromosome regions during recovery was also incomplete. This present study reveals for the first time that the spatial restoration of key regulatory factors like hnRNPs, HP1, or RNA pol II to their pre-stress nuclear targets in cells recovering from thermal stress is dependent upon critical level of the large hsrω-n noncoding RNA. In the absence of their relocation to pre-stress chromosome sites, normal developmental gene activity fails to be restored, which finally results in delayed organismal death.











Similar content being viewed by others
Abbreviations
- hnRNP:
-
Heterogeneous nuclear ribonucleoprotein
- HP1:
-
Heterochromatin protein 1
- MT:
-
Malpighian tubules
- PBS:
-
Phosphate buffered saline
- pol II:
-
RNA polymerase II
- PSS:
-
Poels’ salt solution
References
Adhikari AS, Sridhar Rao K, Rangaraj N, Parnaik VK, Mohan Rao C (2004) Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell Res 299:393–403
Ameyar-Zazoua M, Souidi M, Fritsch L, Robin P, Thomas A, Hamiche A, Percipalle P, Ait-Si-Ali S, Harel-Bellan A (2009) Physical and functional interaction between heterochromatin protein 1alpha and the RNA-binding protein heterogeneous nuclear ribonucleoprotein U. J Biol Chem 284:27974–27979
Andrulis ED, Guzman E, Doring P, Werner J, Lis JT (2000) High-resolution localization of Drosophila Spt5 and Spt6 at heat shock genes in vivo: roles in promoter proximal pausing and transcription elongation. Genes Dev 14:2635–2649
Banerjee S, Lee J, Venkatesh K, Wu CF, Hasan G (2004) Loss of flight and associated neuronal rhythmicity in inositol 1,4,5-trisphosphate receptor mutants of Drosophila. J Neurosci 24:7869–7878
Bendena WG, Garbe JC, Traverse KL, Lakhotia SC, Pardue ML (1989) Multiple inducers of the Drosophila heat shock locus 93D (hsr omega): inducer-specific patterns of the three transcripts. J Cell Biol 108:2017–2028
Bendena WG, Ayme-Southgate A, Garbe JC, Pardue ML (1991) Expression of heat-shock locus hsr-omega in nonstressed cells during development in Drosophila melanogaster. Dev Biol 144:65–77
Bergh S, Arking R (1984) Developmental profile of the heat shock response in early embryos of Drosophila. J Exp Zool 231:379–391
Biamonti G, Vourc’h C (2010) Nuclear stress bodies. Cold Spring Harb Perspect Biol 2:a000695
Boehm AK, Saunders A, Werner J, Lis JT (2003) Transcription factor and polymerase recruitment, modification, and movement on dhsp70 in vivo in the minutes following heat shock. Mol Cell Biol 23:7628–7637
Bomsztyk K, Denisenko O, Ostrowski J (2004) hnRNP K: one protein multiple processes. Bioessays 26:629–638
Bonner JJ, Kerby RL (1982) RNA polymerase II transcribes all of the heat shock induced genes of Drosophila melanogaster. Chromosoma 85:93–108
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415
Bregman DB, Du L, Vanderzee S, Warren SL (1995) Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J Cell Biol 129:287–298
Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, Wilhelm JE, Murphy TD, Levis RW, Matunis E, Srivali N, Hoskins RA, Spradling AC (2007) The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175:1505–1531
Chaudhury A, Chander P, Howe PH (2010) Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: focus on hnRNP E1’s multifunctional regulatory roles. RNA 16:1449–1462
Dangli A, Bautz EK (1983) Differential distribution of nonhistone proteins from polytene chromosomes of Drosophila melanogaster after heat shock. Chromosoma 88:201–207
Dangli A, Grond C, Kloetzel P, Bautz EK (1983) Heat-shock puff 93 D from Drosophila melanogaster: accumulation of a RNP-specific antigen associated with giant particles of possible storage function. Embo J 2:1747–1751
Espinoza CA, Allen TA, Hieb AR, Kugel JF, Goodrich JA (2004) B2 RNA binds directly to RNA polymerase II to repress transcript synthesis. Nature Struct Mol Biol 11:822–829
Evgen’ev MB, Garbuz DG, Shilova VY, Zatsepina OG (2007) Molecular mechanisms underlying thermal adaptation of xeric animals. J Biosciences 32:489–499
Garbe JC, Bendena WG, Alfano M, Pardue ML (1986) A Drosophila heat shock locus with a rapidly diverging sequence but a conserved structure. J Biol Chem 261:16889–16894
Gattoni R, Mahe D, Mahl P, Fischer N, Mattei MG, Stevenin J, Fuchs JP (1996) The human hnRNP-M proteins: structure and relation with early heat shock-induced splicing arrest and chromosome mapping. Nucleic Acids Res 24:2535–2542
Gerber M, Tenney K, Conaway JW, Conaway RC, Eissenberg JC, Shilatifard A (2005) Regulation of heat shock gene expression by RNA polymerase II elongation factor, elongin A. J Biol Chem 280:4017–4020
Giordano E, Rendina R, Peluso I, Furia M (2002) RNAi triggered by symmetrically transcribed transgenes in Drosophila melanogaster. Genetics 160:637–648
Han SP, Kassahn KS, Skarshewski A, Ragan MA, Rothnagel JA, Smith R (2010) Functional implications of the emergence of alternative splicing in hnRNP A/B transcripts. RNA 16:1760–1768
Handwerger KE, Gall JG (2006) Subnuclear organelles: new insights into form and function. Trends Cell Biol 16:19–26
Handwerger KE, Wu Z, Murphy C, Gall JG (2002) Heat shock induces mini-Cajal bodies in the Xenopus germinal vesicle. J Cell Sci 115:2011–2020
Haynes SR, Johnson D, Raychaudhuri G, Beyer AL (1991) The Drosophila Hrb87F gene encodes a new member of the A and B hnRNP protein group. Nucleic Acids Res 19:25–31
Ji Y, Tulin AV (2009) Poly(ADP-ribosyl)ation of heterogeneous nuclear ribonucleoproteins modulates splicing. Nucleic Acids Res 37:3501–3513
Johnson TK, Cockerell FE, McKechnie SW (2011) Transcripts from the Drosophila heat-shock gene hsr-omega influence rates of protein synthesis but hardly affect resistance to heat knockdown. Mol Genet Genomics 285:313–323
Jolly C, Lakhotia SC (2006) Human sat III and Drosophila hsr omega transcripts: a common paradigm for regulation of nuclear RNA processing in stressed cells. Nucleic Acids Res 34:5508–5514
Kim MK, Nikodem VM (1999) hnRNP U inhibits carboxy-terminal domain phosphorylation by TFIIH and represses RNA polymerase II elongation. Mol Cell Biol 19:6833–6844
Lakhotia SC (1989) The 93D heat-shock locus of Drosophila melanogaster - modulation by genetic and developmental factors. Genome 31:677–683
Lakhotia SC (2003) The noncoding developmentally active and stress inducible hsr gene of Drosophila melanogaster integrates post-transcriptional processing of other nuclear transcripts. In: Barciszewski J, Erdmann VA (eds) Noncoding RNAs: molecular biology and molecular medicine. Kluwer Academic/Plenum Publishers, New York, pp 203–221
Lakhotia SC (2011) Forty years of the 93D puff of Drosophila melanogaster. J Biosci 36:399–423
Lakhotia SC, Mukherjee T (1982) Absence of novel translation products in relation to induced activity of the 93D puff in Drosophila melanogaster. Chromosoma 85:369–374
Lakhotia SC, Tapadia MG (1998) Genetic mapping of the amide response element(s) of the hsr omega locus of Drosophila melanogaster. Chromosoma 107:127–135
Lakhotia SC, Ray P, Rajendra TK, Prasanth KV (1999) The noncoding transcripts of hsr-omega gene in Drosophila: do they regulate trafficking and availability of nuclear RNA-processing factors? Curr Sci 77:553–563
Lakhotia SC, Rajendra TK, Prasanth KV (2001) Developmental regulation and complex organization of the promoter of the noncoding hsr(omega) gene of Drosophila melanogaster. J Biosciences 26:25–38
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191
Lis JT (2007) Imaging Drosophila gene activation and polymerase pausing in vivo. Nature 450:198–202
Lis JT, Mason P, Peng J, Price DH, Werner J (2000) P-TEFb kinase recruitment and function at heat shock loci. Genes Dev 14:792–803
Mallik M, Lakhotia SC (2009a) RNAi for the large noncoding hsromega transcripts suppresses polyglutamine pathogenesis in Drosophila models. RNA Biol 6:464–478
Mallik M, Lakhotia SC (2009b) The developmentally active and stress-inducible noncoding hsromega gene is a novel regulator of apoptosis in Drosophila. Genetics 183:831–852
Mallik M, Lakhotia SC (2010) Improved activities of CREB binding protein, heterogeneous nuclear ribonucleoproteins and proteasome following downregulation of noncoding hsr transcripts help suppress poly(Q) pathogenesis in fly models. Genetics 184:927–945
Mallik M, Lakhotia SC (2011) Misexpression of the developmentally active and stress-inducible noncoding hsrω gene in Drosophila has pleiotropic consequences. J Biosci 36:265–280
Morimoto RI (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22:1427–1438
Morin X, Daneman R, Zavortink M, Chia W (2001) A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci USA 98:15050–15055
Mukherjee T, Lakhotia SC (1979) 3H-uridine incorporation in the puff 93D and in chromocentric heterochromatin of heat shocked salivary glands of Drosophila melanogaster. Chromosoma 74:75–82
Mutsuddi M, Lakhotia SC (1995) Spatial expression of the hsr-omega (93D) gene in different tissues of Drosophila melanogaster and identification of promoter elements controlling its developmental expression. Dev Genet 17:303–311
Onorati MC, Lazzaro S, Mallik M, Ingrassia AMR, Singh AK, Chaturvedi DP, Lakhotia SC, Corona DFV (2011) The ISWI chromatin remodeler organizes the hsrω ncRNA-containing omega speckle nuclear compartments. PLoS Genet 7:e1002096. doi:10.1371/journal.pgen.1002096
Piacentini L, Fanti L, Berloco M, Perrini B, Pimpinelli S (2003) Heterochromatin protein 1 (HP1) is associated with induced gene expression in Drosophila euchromatin. J Cell Biol 161:707–714
Piacentini L, Fanti L, Negri R, Del Vescovo V, Fatica A, Altieri F, Pimpinelli S (2009) Heterochromatin protein 1 (HP1a) positively regulates euchromatic gene expression through RNA transcript association and interaction with hnRNPs in Drosophila. PLoS Genetics 5:e1000670
Prasanth KV, Rajendra TK, Lal AK, Lakhotia SC (2000) Omega speckles—a novel class of nuclear speckles containing hnRNPs associated with noncoding hsr-omega RNA in Drosophila. J Cell Sci 113:3485–3497
Rorth P (1996) A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc Natl Acad Sci USA 93:12418–12422
Saumweber H, Symmons P, Kabisch R, Will H, Bonhoeffer F (1980) Monoclonal antibodies against chromosomal proteins of Drosophila melanogaster: establishment of antibody producing cell lines and partial characterization of corresponding antigens. Chromosoma 80:253–275
Sengupta S, Lakhotia SC (2006) Altered expressions of the noncoding hsromega gene enhances poly-Q-induced neurotoxicity in Drosophila. RNA Biol 3:28–35
Shamovsky I, Nudler E (2008) Modular RNA heats up. Molec Cell 29:415–417
Spector DL, Lamond AI (2010) Nuclear speckles. Cold Spring Harbor Perspect Biol. doi:10.1101/cshperspect.a000646
Tapadia MG, Lakhotia SC (1997) Specific induction of the hsr omega locus of Drosophila melanogaster by amides. Chromosome Res 5:359–362
Tulin A, Spradling A (2003) Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science 299:560–562
Velazquez JM, Lindquist S (1984) Hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell 36:655–662
Yakovchuk P, Goodrich JA, Kugel JF (2009) B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci USA 106:5569–5574
Zeng C, Kim E, Warren SL, Berget SM (1997) Dynamic relocation of transcription and splicing factors dependent upon transcriptional activity. Embo J 16:1401–1412
Acknowledgments
We thank Dr. Gaiti Hasan (NCBS, Bangalore, India) and Dr. A. Spradling (Baltimore, USA) for providing the hs-GAL4 t /CyO and the w; Hrb87F-GFP/Hrb87F-GFP fly stocks, respectively. We acknowledge the kind gift of the P11 antibody by Dr. H. Saumweber (Berlin, Germany) and 7Fb antibody by Dr. M. B. Evgen’ev (Russia). The work was supported by the Department of Science and Technology, Govt. of India (New Delhi) through the Ramanna Fellowship and the National Facility for Confocal Microscopy grants to SCL. MM was supported by the Shyama Prasad Mukherjee fellowship of the Council of Scientific and Industrial Research (CSIR, New Delhi) while AKS and MR are recipients of Research Fellowships from the CSIR.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00412-011-0350-9.
Rights and permissions
About this article
Cite this article
Lakhotia, S.C., Mallik, M., Singh, A.K. et al. The large noncoding hsrω-n transcripts are essential for thermotolerance and remobilization of hnRNPs, HP1 and RNA polymerase II during recovery from heat shock in Drosophila . Chromosoma 121, 49–70 (2012). https://doi.org/10.1007/s00412-011-0341-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-011-0341-x