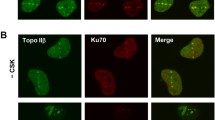
Abstract
Human DNA topoisomerase I is an essential enzyme involved in resolving the torsional stress associated with DNA replication, transcription, and chromatin condensation. The catalytic cycle of the enzyme consists of DNA cleavage to form a covalent enzyme–DNA intermediate, DNA relaxation, and finally, religation of the phosphate backbone to restore the continuity of the DNA. Structure/function studies have elucidated a flexible enzyme that relaxes DNA through coordinated, controlled movements of distinct enzyme domains. The cellular roles of topoisomerase I are apparent throughout the nucleus, but the concentration of processes acting on ribosomal DNA results in topoisomerase I accumulation in the nucleolus. Although the activity of topoisomerase I is required in these processes, the enzyme can also have a deleterious effect on cells. In the event that the final religation step of the reaction cycle is prevented, the covalent topoisomerase I–DNA intermediate becomes a toxic DNA lesion that must be repaired. The complexities of the relaxation reaction, the cellular roles, and the pathways that must exist to repair topoisomerase I-mediated DNA damage highlight the importance of continued study of this essential enzyme.





Similar content being viewed by others
References
Alsner J, Svejstrup JQ, Kjeldsen E, Sorensen BS, Westergaard O (1992) Identification of an N-terminal domain of eukaryotic DNA topoisomerase I dispensable for catalytic activity but essential for in vivo function. J Biol Chem 267:12408–12411
Arabi A, Rustum C, Hallberg E, Wright AP (2003) Accumulation of c-Myc and proteasomes at the nucleoli of cells containing elevated c-Myc protein levels. J Cell Sci 116:1707–1717
Ayaydin F, Dasso M (2004) Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol Biol Cell 15:5208–5218
Baker SD, Wadkins RM, Stewart CF, Beck WT, Danks MK (1995) Cell cycle analysis of amount and distribution of nuclear DNA topoisomerase I as determined by fluorescence digital imaging microscopy. Cytometry 19:134–145
Barrows LR, Holden JA, Anderson M, D’Arpa P (1998) The CHO XRCC1 mutant, EM9, deficient in DNA ligase III activity, exhibits hypersensitivity to camptothecin independent of DNA replication. Mutat Res 408:103–110
Bauer PI, Buki KG, Comstock JA, Kun E (2000) Activation of topoisomerase I by poly [ADP-ribose] polymerase. Int J Mol Med 5:533–540
Been MD, Burgess RR, Champoux JJ (1984) Nucleotide sequence preference at rat liver and wheat germ type 1 DNA topoisomerase breakage sites in duplex SV40 DNA. Nucleic Acids Res 12:3097–3114
Bharti AK, Olson MO, Kufe DW, Rubin EH (1996) Identification of a nucleolin binding site in human topoisomerase I. J Biol Chem 271:P1993–P1997
Bjornsti MA, Benedetti P, Viglianti GA, Wang JC (1989) Expression of human DNA topoisomerase I in yeast cells lacking yeast DNA topoisomerase I: restoration of sensitivity of the cells to the antitumor drug camptothecin. Cancer Res 49:6318–6323
Bonven BJ, Gocke E, Westergaard O (1985) A high affinity topoisomerase I binding sequence is clustered at DNAase I hypersensitive sites in Tetrahymena R-chromatin. Cell 41:541–551
Buckwalter CA, Lin AH, Tanizawa A, Pommier YG, Cheng YC, Kaufmann SH (1996) RNA synthesis inhibitors alter the subnuclear distribution of DNA topoisomerase I. Cancer Res 56:1674–1681
Carey JF, Schultz SJ, Sisson L, Fazzio TG, Champoux JJ (2003) DNA relaxation by human topoisomerase I occurs in the closed clamp conformation of the protein. Proc Natl Acad Sci U S A 100:5640–5645
Castano IB, Brzoska PM, Sadoff BU, Chen H, Christman MF (1996) Mitotic chromosome condensation in the rDNA requires TRF4 and DNA topoisomerase I in Saccharomyces cerevisiae. Genes Dev 10:2564–2576
Catley L, Tai YT, Shringarpure R, Burger R, Son MT, Podar K, Tassone P, Chauhan D, Hideshima T, Denis L, Richardson P, Munshi NC, Anderson KC (2004) Proteasomal degradation of topoisomerase I is preceded by c-Jun NH2-terminal kinase activation, Fas up-regulation, and poly(ADP-ribose) polymerase cleavage in SN38-mediated cytotoxicity against multiple myeloma. Cancer Res 64:8746–8753
Champoux J (1990) DNA topology and its biological effects. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 217–242
Champoux JJ (2001) DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 70:369–413
Champoux JJ, Dulbecco R (1972) An activity from mammalian cells that untwists superhelical DNA—a possible swivel for DNA replication (polyoma–ethidium bromide–mouse–embryo cells–dye binding assay). Proc Natl Acad Sci U S A 69:143–146
Chillemi G, Redinbo M, Bruselles A, Desideri A (2004) Role of the linker domain and the 203–214 N-terminal residues in the human topoisomerase I DNA complex dynamics. Biophys J 87:4087–4097
Christensen MO, Barthelmes HU, Boege F, Mielke C (2002a) The N-terminal domain anchors human topoisomerase I at fibrillar centers of nucleoli and nucleolar organizer regions of mitotic chromosomes. J Biol Chem 277:35932–35938
Christensen MO, Barthelmes HU, Feineis S, Knudsen BR, Andersen AH, Boege F, Mielke C (2002b) Changes in mobility account for camptothecin-induced subnuclear relocation of topoisomerase I. J Biol Chem 277:15661–15665
Christensen MO, Barthelmes HU, Boege F, Mielke C (2003) Residues 190–210 of human topoisomerase I are required for enzyme activity in vivo but not in vitro. Nucleic Acids Res 31:7255–7263
Christensen MO, Krokowski RM, Barthelmes HU, Hock R, Boege F, Mielke C (2004) Distinct effects of topoisomerase I and RNA polymerase I inhibitors suggest a dual mechanism of nucleolar/nucleoplasmic partitioning of topoisomerase I. J Biol Chem 279:21873–21882
Connelly JC, Leach DR (2004) Repair of DNA covalently linked to protein. Mol Cell 13:307–316
Danks MK, Garrett KE, Marion RC, Whipple DO (1996) Subcellular redistribution of DNA topoisomerase I in anaplastic astrocytoma cells treated with topotecan. Cancer Res 56:1664–1673
Debethune L, Kohlhagen G, Grandas A, Pommier Y (2002) Processing of nucleopeptides mimicking the topoisomerase I–DNA covalent complex by tyrosyl–DNA phosphodiesterase. Nucleic Acids Res 30:1198–1204
Desai SD, Liu LF, Vazquez-Abad D, D’Arpa P (1997) Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J Biol Chem 272:24159–24164
Desai SD, Zhang H, Rodriguez-Bauman A, Yang JM, Wu X, Gounder MK, Rubin EH, Liu LF (2003) Transcription-dependent degradation of topoisomerase I–DNA covalent complexes. Mol Cell Biol 23:2341–2350
El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW (2005) Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434:108–113
Frohlich RF, Andersen FF, Westergaard O, Andersen AH, Knudsen BR (2004) Regions within the N-terminal domain of human topoisomerase I exert important functions during strand rotation and DNA binding. J Mol Biol 336:93–103
Gao H, Chen XB, McGowan CH (2003) Mus81 endonuclease localizes to nucleoli and to regions of DNA damage in human S-phase cells. Mol Biol Cell 14:4826–4834
Garcia-Carbonero R, Supko JG (2002) Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res 8:641–661
Gobert C, Bracco L, Rossi F, Olivier M, Tazi J, Lavelle F, Larsen AK, Riou JF (1996) Modulation of DNA topoisomerase I activity by p53. Biochemistry 35:5778–5786
Gonzalez IL, Sylvester JE (1995) Complete sequence of the 43-kb human ribosomal DNA repeat: analysis of the intergenic spacer. Genomics 27:320–328
Haluska P Jr, Saleem A, Rasheed Z, Ahmed F, Su EW, Liu LF, Rubin EH (1999) Interaction between human topoisomerase I and a novel RING finger/arginine–serine protein. Nucleic Acids Res 27:2538–2544
Hannan KM, Hannan RD, Rothblum LI (1998) Transcription by RNA polymerase I. Front Biosci 3:d376–d398
Hsiang YH, Liu LF (1988) Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48:1722–1726
Interthal H, Pouliot JJ, Champoux JJ (2001) The tyrosyl–DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc Natl Acad Sci U S A 98:12009–12014
Interthal H, Quigley PM, Hol WG, Champoux JJ (2004) The role of lysine 532 in the catalytic mechanism of human topoisomerase I. J Biol Chem 279:2984–2992
Karayan L, Riou JF, Seite P, Migeon J, Cantereau A, Larsen CJ (2001) Human ARF protein interacts with topoisomerase I and stimulates its activity. Oncogene 20:836–848
Kretzschmar M, Meisterernst M, Roeder RG (1993) Identification of human DNA topoisomerase I as a cofactor for activator-dependent transcription by RNA polymerase II. Proc Natl Acad Sci U S A 90:11508–11512
Laine JP, Opresko PL, Indig FE, Harrigan JA, von Kobbe C, Bohr VA (2003) Werner protein stimulates topoisomerase I DNA relaxation activity. Cancer Res 63:7136–7146
Lebel M, Spillare EA, Harris CC, Leder P (1999) The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J Biol Chem 274:37795–37799
Lee MP, Brown SD, Chen A, Hsieh TS (1993) DNA topoisomerase I is essential in Drosophila melanogaster. Proc Natl Acad Sci U S A 90:6656–6660
Leppard JB, Dong Z, Mackey ZB, Tomkinson AE (2003) Physical and functional interaction between DNA ligase IIIalpha and poly(ADP-ribose) polymerase 1 in DNA single-strand break repair. Mol Cell Biol 23:5919–5927
Lesher DT, Pommier Y, Stewart L, Redinbo MR (2002) 8-Oxoguanine rearranges the active site of human topoisomerase I. Proc Natl Acad Sci U S A 99:12102–12107
Li TK, Liu LF (2001) Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol 41:53–77
Liu C, Pouliot JJ, Nash HA (2002) Repair of topoisomerase I covalent complexes in the absence of the tyrosyl–DNA phosphodiesterase Tdp1. Proc Natl Acad Sci U S A 99:14970–14975
Madden KR, Stewart L, Champoux JJ (1995) Preferential binding of human topoisomerase I to superhelical DNA. EMBO J 14:5399–5409
Mao Y, Muller MT (2003) Down modulation of topoisomerase I affects DNA repair efficiency. DNA Repair (Amst) 2:1115–1126
Mao Y, Sun M, Desai SD, Liu LF (2000) SUMO-1 conjugation to topoisomerase I: a possible repair response to topoisomerase-mediated DNA damage. Proc Natl Acad Sci U S A 97:4046–4051
Mao Y, Mehl IR, Muller MT (2002) Subnuclear distribution of topoisomerase I is linked to ongoing transcription and p53 status. Proc Natl Acad Sci U S A 99:1235–1240
Meder VS, Boeglin M, de Murcia G, Schreiber V (2005) PARP-1 and PARP-2 interact with nucleophosmin/B23 and accumulate in transcriptionally active nucleoli. J Cell Sci 118:211–222
Merino A, Madden KR, Lane WS, Champoux JJ, Reinberg D (1993) DNA topoisomerase I is involved in both repression and activation of transcription. Nature 365:227–232
Mo YY, Wang C, Beck WT (2000) A novel nuclear localization signal in human DNA topoisomerase I. J Biol Chem 275:41107–41113
Morham SG, Kluckman KD, Voulomanos N, Smithies O (1996) Targeted disruption of the mouse topoisomerase I gene by camptothecin selection. Mol Cell Biol 16:6804–6809
Morris EJ, Geller HM (1996) Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J Cell Biol 134:757–770
Muller MT, Pfund WP, Mehta VB, Trask DK (1985) Eukaryotic type I topoisomerase is enriched in the nucleolus and catalytically active on ribosomal DNA. EMBO J 4:1237–1243
Okano S, Lan L, Caldecott KW, Mori T, Yasui A (2003) Spatial and temporal cellular responses to single-strand breaks in human cells. Mol Cell Biol 23:3974–3981
Plo I, Liao ZY, Barcelo JM, Kohlhagen G, Caldecott KW, Weinfeld M, Pommier Y (2003) Association of XRCC1 and tyrosyl DNA phosphodiesterase (Tdp1) for the repair of topoisomerase I-mediated DNA lesions. DNA Repair (Amst) 2:1087–1100
Pommier Y, Cherfils J (2005) Interfacial inhibition of macromolecular interactions: nature’s paradigm for drug discovery. Trends Pharmacol Sci 26:138–145
Pommier Y, Pourquier P, Fan Y, Strumberg D (1998) Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim Biophys Acta 1400:83–105
Pommier Y, Redon C, Rao VA, Seiler JA, Sordet O, Takemura H, Antony S, Meng L, Liao Z, Kohlhagen G, Zhang H, Kohn KW (2003) Repair of and checkpoint response to topoisomerase I-mediated DNA damage. Mutat Res 532:173–203
Pouliot JJ, Yao KC, Robertson CA, Nash HA (1999) Yeast gene for a Tyr–DNA phosphodiesterase that repairs topoisomerase I complexes. Science 286:552–555
Pouliot JJ, Robertson CA, Nash HA (2001) Pathways for repair of topoisomerase I covalent complexes in Saccharomyces cerevisiae. Genes Cells 6:677–687
Rallabhandi P, Hashimoto K, Mo YY, Beck WT, Moitra PK, D’Arpa P (2002) Sumoylation of topoisomerase I is involved in its partitioning between nucleoli and nucleoplasm and its clearing from nucleoli in response to camptothecin. J Biol Chem 277:40020–40026
Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG (1998) Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science 279:1504–1513
Redinbo MR, Stewart L, Champoux JJ, Hol WG (1999) Structural flexibility in human topoisomerase I revealed in multiple non-isomorphous crystal structures. J Mol Biol 292:685–696
Redinbo MR, Champoux JJ, Hol WG (2000) Novel insights into catalytic mechanism from a crystal structure of human topoisomerase I in complex with DNA. Biochemistry 39:6832–6840
Rossi F, Labourier E, Forne T, Divita G, Derancourt J, Riou JF, Antoine E, Cathala G, Brunel C, Tazi J (1996) Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 381:80–82
Rubbi CP, Milner J (2003) Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J 22:6068–6077
Schwarzacher HG, Wachtler F (1993) The nucleolus. Anat Embryol (Berl) 188:515–536
Seeler JS, Dejean A (2003) Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol 4:690–699
Sokhansanj BA, Wilson DM III (2004) Oxidative DNA damage background estimated by a system model of base excision repair. Free Radic Biol Med 37:422–427
Sordet O, Khan QA, Plo I, Pourquier P, Urasaki Y, Yoshida A, Antony S, Kohlhagen G, Solary E, Saparbaev M, Laval J, Pommier Y (2004) Apoptotic topoisomerase I–DNA complexes induced by staurosporine-mediated oxygen radicals. J Biol Chem 279:50499–50504
Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB Jr, Stewart L (2002) The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A 99:15387–15392
Stewart L, Ireton GC, Champoux JJ (1997) Reconstitution of human topoisomerase I by fragment complementation. J Mol Biol 269:355–372
Stewart L, Redinbo MR, Qiu X, Hol WG, Champoux JJ (1998) A model for the mechanism of human topoisomerase I. Science 279:1534–1541
Stewart L, Ireton GC, Champoux JJ (1999) A functional linker in human topoisomerase I is required for maximum sensitivity to camptothecin in a DNA relaxation assay. J Biol Chem 274:32950–32960
Straub T, Grue P, Uhse A, Lisby M, Knudsen BR, Tange TO, Westergaard O, Boege F (1998) The RNA-splicing factor PSF/p54 controls DNA-topoisomerase I activity by a direct interaction. J Biol Chem 273:26261–26264
Strumberg D, Pilon AA, Smith M, Hickey R, Malkas L, Pommier Y (2000) Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol Cell Biol 20:3977–3987
Tanizawa A, Kohn KW, Pommier Y (1993) Induction of cleavage in topoisomerase I c-DNA by topoisomerase I enzymes from calf thymus and wheat germ in the presence and absence of camptothecin. Nucleic Acids Res 21:5157–5166
Vance JR, Wilson TE (2002) Yeast Tdp1 and Rad1–Rad10 function as redundant pathways for repairing Top1 replicative damage. Proc Natl Acad Sci U S A 99:13669–13674
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604
Wang JC (1985) DNA topoisomerases. Annu Rev Biochem 54:665–697
Wang JC (1996) DNA topoisomerases. Annu Rev Biochem 65:635–692
Wang JC (2002) Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol 3:430–440
Woo MH, Losasso C, Guo H, Pattarello L, Benedetti P, Bjornsti MA (2003) Locking the DNA topoisomerase I protein clamp inhibits DNA rotation and induces cell lethality. Proc Natl Acad Sci U S A 100:13767–13772
Wu Y, Hickey R, Lawlor K, Wills P, Yu F, Ozer H, Starr R, Quan JY, Lee M, Malkas L (1994) A 17S multiprotein form of murine cell DNA polymerase mediates polyomavirus DNA replication in vitro. J Cell Biochem 54:32–46
Yang SW, Burgin AB Jr, Huizenga BN, Robertson CA, Yao KC, Nash HA (1996) A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Natl Acad Sci U S A 93:11534–11539
Zechiedrich EL, Osheroff N (1990) Eukaryotic topoisomerases recognize nucleic acid topology by preferentially interacting with DNA crossovers. EMBO J 9:4555–4562
Zhang H, Wang JC, Liu LF (1988) Involvement of DNA topoisomerase I in transcription of human ribosomal RNA genes. Proc Natl Acad Sci U S A 85:1060–1064
Zhang CX, Chen AD, Gettel NJ, Hsieh TS (2000) Essential functions of DNA topoisomerase I in Drosophila melanogaster. Dev Biol 222:27–40
Zhang H, Barcelo JM, Lee B, Kohlhagen G, Zimonjic DB, Popescu NC, Pommier Y (2001) Human mitochondrial topoisomerase I. Proc Natl Acad Sci U S A 98:10608–10613
Zhang H, Meng LH, Zimonjic DB, Popescu NC, Pommier Y (2004) Thirteen-exon-motif signature for vertebrate nuclear and mitochondrial type IB topoisomerases. Nucleic Acids Res 32:2087–2092
Acknowledgements
We thank Sharon Schultz and Heidrun Interthal for insightful comments during the preparation of the manuscript. This work was supported by grants GM60330 and GM49156 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. A. Nigg
Rights and permissions
About this article
Cite this article
Leppard, J.B., Champoux, J.J. Human DNA topoisomerase I: relaxation, roles, and damage control. Chromosoma 114, 75–85 (2005). https://doi.org/10.1007/s00412-005-0345-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-005-0345-5