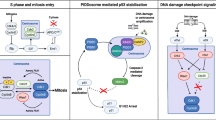
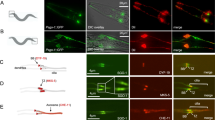
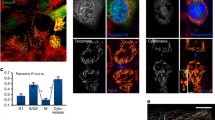
Abstract
Cenp-F (mitosin) is a large coiled-coil protein whose function has remained obscure since its identification a decade ago. It has been suggested that the protein plays a role in the kinetochore-mediated mitotic functions but until recently there was little evidence to support this postulation. Recent results from five laboratories have given insights on how Cenp-F may participate in the regulation of cell division. In this mini-review, we will summarize the current data regarding the mitotic tasks of Cenp-F as well as discuss how it is used as a proliferation marker of malignant cell growth in the clinic. Also, the protein’s post-translational modification by farnesylation and potential contribution to cell cycle effects of farnesyl transferase inhibitors will be addressed.


Similar content being viewed by others
References
Ashar HR, James L, Gray K, Carr D, Black S, Armstrong L, Bishop WR, Kirschmeier P (2000) Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem 275:30451–30457
Bishop WR, Kirschmeier P, Baum C (2003) Farnesyl transferase inhibitors: mechanism of action, translational studies and clinical evaluation. Cancer Biol Ther 2(Suppl 1):S96–S104
Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW (2005) Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J 24:3927–3939
Carvalho P, Tirnauer JS, Pellman D (2003) Surfing on microtubule ends. Trends Cell Biol 13:229–237
Casiano CA, Humbel RL, Peebles C, Covini G, Tan EM (1995) Autoimmunity to the cell cycle-dependent centromere protein p330d/CENP-F in disorders associated with cell proliferation. J Autoimmun 8:575–586
Casiano CA, Landberg G, Ochs RL, Tan EM (1993) Autoantibodies to a novel cell cycle-regulated protein that accumulates in the nuclear matrix during S phase and is localized in the kinetochores and spindle midzone during mitosis. J Cell Sci 106:1045–1056
Chan GKT, Schaar BT, Yen TJ (1998) Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J Cell Biol 143:49–63
Chan GK, Yen TJ (2003) The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit. Prog Cell Cycle Res 5:431–439
Cheeseman IM, MacLeod I, Yates JR 3rd, Oegema K, Desai A (2005) The CENP-F-like proteins HCP-1 and HCP-2 target CLASP to kinetochores to mediate chromosome segregation. Curr Biol 15:771–777
Clark GM, Allred DC, Hilsenbeck SG, Chamness GC, Osborne CK, Jones D, Lee WH (1997) Mitosin (a new proliferation marker) correlates with clinical outcome in node-negative breast cancer. Cancer Res 57:5505–5508
Cleveland DW, Mao Y, Sullivan KF (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signalling. Cell 112:407–421
Crespo NC, Ohkanda J, Yen TJ, Hamilton AD, Sebti SM (2001) The farnesyltransferase inhibitor, FTI-2153, blocks bipolar spindle formation and chromosome alignment and causes prometaphase accumulation during mitosis of human lung cancer cells. J Biol Chem 276:16161–16167
Ducat D, Zheng Y (2004) Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res 301:60–67
Earnshaw WC, Bernat RL (1991) Chromosomal passengers: toward an integrated view of mitosis. Chromosoma 100:139–146
Earnshaw WC, Rothfield N (1985) Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91:313–321
Encalada SE, Willis J, Lyczak R, Bowerman B (2005) A spindle checkpoint functions during mitosis in the early Caenorhabditis elegans embryo. Mol Biol Cell 16:1056–1070
Erlanson M, Casiano CA, Tan EM, Lindh J, Roos G, Landberg G (1999) Immunohistochemical analysis of the proliferation associated nuclear antigen CENP-F in non-Hodgkin’s lymphoma. Mod Pathol 12:69–74
Feng J, Huang H, Yen TJ (2006) CENP-F is a novel microtubule binding protein that is essential for kinetochore attachments and contributes to the mitotic checkpoint delay. Chromosoma (in press).
Forozan F, Mahlamaki EH, Monni O, Chen Y, Veldman R, Jiang Y, Gooden GC, Ethier SP, Kallioniemi A, Kallioniemi OP (2000) Comparative genomic hybridization analysis of 38 breast cancer cell lines: a basis for interpreting complementary DNA microarray data. Cancer Res 60:4519–4525
Fukagawa T (2004) Assembly of kinetochores in vertebrate cells. Exp Cell Res 296:21–27
de la Guardia C, Casiano CA, Trinidad-Pinedo J, Baez A (2001) CENP-F gene amplification and overexpression in head and neck squamous cell carcinomas. Head Neck 23:104–112
Holt SV, Vergnolle MAS, Hussein D, Wozniak MJ, Allan VJ, Taylor SS (2005) Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci 118:4889–4900
Hui D, Reiman T, Hanson J, Linford R, Wong W, Belch A, Lai R (2005) Immunohistochemical detection of cdc2 is useful in predicting survival in patients with mantle cell lymphoma. Mod Pathol 18:1223–1231
Hussein D, Taylor SS (2002) Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci 115:3403–3414
Jablonski SA, Chan GK, Cooke CA, Earnshaw WC, Yen TJ (1998) The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma 107:386–396
Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS (2004) Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci 117:1577–1589
Kitajima TS, Hauf S, Ohsugi M, Yamamoto T, Watanabe Y (2005) Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr Biol 15:353–359
Koon N, Schneider-Stock R, Sarlomo-Rikala M, Lasota J, Smolkin M, Petroni G, Zaika A, Boltze C, Meyer F, Andersson L, Knuutila S, Miettinen M, El-Rifai W (2004) Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut 53:235–240
Landberg G, Erlanson M, Roos G, Tan EM, Casiano CA (1996) Nuclear autoantigen p330d/CENP-F: a marker for cell proliferation in human malignancies. Cytometry 25:90–98
Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH (2005) FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 7:126–136
Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ (1995) CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol 130:507–518
Liu ST, Hittle JC, Jablonski SA, Campbell MS, Yoda K, Yen, TJ (2003) Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nat Cell Biol 5:341–345
Liu SC, Klein-Szanto AJ (1998) Markers of cell proliferation in normal epithelia and dysplastic leukoplakias of the oral cavity. Cancer Epidemiol Biomarkers Prev 7:597–603
Maiato H, DeLuca J, Salmon ED, Earnshaw WC (2004) The dynamic kinetochore-microtubule interface. J Cell Sci 117:5461–5477
Maiato H, Khodjakov A, Rieder CL (2005) Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibres. Nat Cell Biol 7:42–47
McGuinness BE, Hirota T, Kudo NR, Peters J-M, Nasmyth K (2005) Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol 3:e86
Meraldi P, Honda R, Nigg EA (2002) Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J 21:483–492
Meraldi P, Sørger PK (2005) A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J 24:1621–1633
Mijimolle N, Velasco J, Dubus P, Guerra C, Weinbaum CA, Casey PJ, Campuzano V, Barbacid M (2005) Protein farnesyltransferase in embryogenesis, adult homeostasis, and tumor development. Cancer Cell 7:313–324
Moore LL, Morrison M, Roth MB (1999) HCP-1, a protein involved in chromosome segregation, is localized to the centromere of mitotic chromosomes in Caenorhabditis elegans. J Cell Biol 147:471–480
O’Regan RM, Khuri FR (2004) Farnesyl transferase inhibitors: the next targeted therapies for breast cancer? Endocr Relat Cancer 11:191–205
Pidoux AL, Allshire RC (2000) Centromeres: getting a grip of chromosomes. Curr Opin Cell Biol 12:308–319
Pimkhaokham A, Shimada Y, Fukuda Y, Kurihara N, Imoto I, Yang ZQ, Imamura M, Nakamura Y, Amagasa T, Inazawa J (2000) Nonrandom chromosomal imbalances in esophageal squamous cell carcinoma cell lines: possible involvement of the ATF3 and CENPF genes in the 1q32 amplicon. Jpn J Cancer Res 91:1126–1133
Putkey FR, Cramer T, Morphew MK, Silk AD, Johnson RS, McIntosh JR, Cleveland DW (2002) Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev Cell 3:351–365
Rattner JB, Rao A, Fritzler MJ, Valencia DW, Yen TJ (1993) CENP-F is a ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil Cytoskeleton 26:214–226
Rattner JB, Rees J, Whitehead CM, Casiano CA, Tan EM, Humbel RL, Conrad K, Fritzler MJ (1997) High frequency of neoplasia in patients with autoantibodies to centromere protein CENP-F. Clin Invest Med 20:308–319
Roskoski R Jr (2003) Protein prenylation: a pivotal posttranslational process. Biochem Biophys Res Commun 303:1–7
Salic A, Waters JC, Mitchison TJ (2004) Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118:567–578
Sebti SM (2005) Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell 7:297–300
Sharma S, Kemeny N, Kelsen DP, Ilson D, O’Reilly E, Zaknoen S, Baum C, Statkevich P, Hollywood E, Zhu Y, Saltz LB (2002) A phase II trial of farnesyl protein transferase inhibitor SCH 66336, given by twice-daily oral administration, in patients with metastatic colorectal cancer refractory to 5-fluorouracil and irinotecan. Ann Oncol 13:1067–1071
Sørensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J (2000) Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol Cell Biol 20:7613–7623
Steensgaard P, Garre M, Muradore I, Transidico P, Nigg EA, Kitagawa K, Earnshaw WC, Faretta M, Musacchio A (2004) Sgt1 is required for human kinetochore assembly. EMBO Rep 5:626–631
Tanudji M, Shoemaker J, L’Italien L, Russell L, Chin G, and Schebye XM (2004) Gene silencing of CENP-E by small interfering RNA in HeLa cells leads to missegregation of chromosomes after a mitotic delay. Mol Biol Cell 15:3771–3781
Taylor SS, Ha E, Mc Keon F (1998) The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J Cell Biol 142:1–11
Taylor SS, Hussein D, Wang Y, Elderkin S, Morrow CJ (2001) Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J Cell Sci 114:4385–4395
Taylor SS, Scott MI, Holland AJ (2004) The spindle checkpoint: a quality control mechanism which ensures accurate chromosome segregation. Chromosome Res 12:599–616
Vagnarelli P, Earnshaw WC (2004) Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma 113:211–222
Wang H, Hu X, Ding X, Dou Z, Yang Z, Shaw AW, Teng M, Cleveland DW, Goldberg ML, Niu L, Yao X (2004) Human Zwint-1 specifies localization of Zeste White 10 to kinetochores and is essential for mitotic checkpoint signaling. J Biol Chem 279:54590–54598
Weaver BA, Bonday ZQ, Putkey FR, Kops GJ, Silk AD, Cleveland DW (2003) Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J Cell Biol 162:551–563
Westendorf JM, Rao PN, Gerace L (1994) Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci U S A 91:714–718
Winter-Vann AM, Casey PJ (2005) Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer 5:405–412
Wonsey DR, Follettie MT (2005) Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res 65:5181–5189
Yang Z, Guo J, Chen Q, Ding C, Du J, Zhu X (2005) Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol Cell Biol 25:4062–4074
Yang ZY, Guo J, Li N, Oian M, Wang SN, Zhu XL (2003) Mitosin/CENP-F is a conserved kinetochore protein subjected to cytoplasmic dynein-mediated poleward transport. Cell Res 13:275–283
Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW (2000) CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat Cell Biol 2:484–491
Zhu X (1999) Structural requirements and dynamics of mitosin–kinetochore interaction in M phase. Mol Cell Biol 19:1016–1024
Zhu X, Chang KH, He D, Mancini MA, Brinkley WR, Lee WH (1995a) The C terminus of mitosin is essential for its nuclear localization, centromere/kinetochore targeting, and dimerization. J Biol Chem 270:19545–19550
Zhu X, Mancini MA, Chang KH, Liu CY, Chen CF, Shan B, Jones D, Yang-Feng TL, Lee WH (1995b) Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol Cell Biol 15:5017–5029
Zhu X, Ding L, Pei G (1997) Carboxyl terminus of mitosin is sufficient to confer spindle pole localization. J Cell Biochem 66:441–449
Acknowledgements
We apologize to our colleagues whose work could not be cited due to space constraints. This work is supported by grants from the Academy of Finland and Marie Curie EXT programme of the European Commission. We are grateful to Gary Gorbsky for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.A. Nigg
Rights and permissions
About this article
Cite this article
Varis, A., Salmela, AL. & Kallio, M.J. Cenp-F (mitosin) is more than a mitotic marker. Chromosoma 115, 288–295 (2006). https://doi.org/10.1007/s00412-005-0046-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-005-0046-0