Abstract
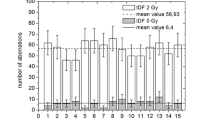
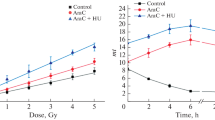
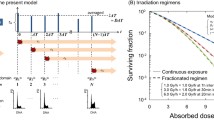
We have studied the dependence of clonogenic bystander effects on defects in the pathways of DNA double-strand break (DSB) repair and on linear energy transfer (LET). The single-ion microbeam of the Physikalisch-Technische Bundesanstalt (PTB) was used to irradiate parental Chinese hamster ovary cells or derivatives deficient in nonhomologous end joining (NHEJ) or homologous recombination (HR) in the G1-phase of the cell cycle. Cell nuclei were targeted with 10 MeV protons (LET = 4.7 keV/μm) or 4.5 MeV α-particles (LET = 100 keV/μm). During exposure, the cells were confluent, allowing signal transfer through both gap junctions and diffusion. When all cell nuclei were targeted with 10 MeV protons, approximately exponential survival curves were obtained for all three cell lines. When only 10% of all cell nuclei were targeted, a significant bystander effect was observed for parental and HR-deficient cells, but not for NHEJ-deficient cells. For all three cell lines, the survival data after exposure of all cell nuclei to 4.5 MeV α-particles could be fitted by exponential curves. When only 10% of all cell nuclei were targeted, significant bystander effects were obtained for parental and HR-deficient cells, whereas for NHEJ-deficient cells a small, but significant, bystander effect was observed only at higher doses. The data suggest that bystander cell killing is a consequence of un- or misrejoined DSB which occur in bystander cells during the S-phase as a result of the processing of oxidative bistranded DNA lesions. The relative contributions of NHEJ and HR to the repairing of DSB in the late S/G2-phase may affect clonogenic bystander effects.


Similar content being viewed by others
References
Mothersill C, Seymour CB (1997) Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol 71:421–427
Sawant S, Zengh W, Hopkins KM, Randers-Pehrson G, Lieberman HB, Hall EJ (2002) The radiation-induced bystander effect for clonogenic survival. Radiat Res 157:361–364
Frankenberg D, Greif K, Giesen U (2006) Radiation response of primary human skin fibroblasts and their bystander cells after exposure to counted particles at low and high LET. Int J Radiat Biol 82:59–67
Lyng FM, Seymour CB, Mothersill C (2000) Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer 83:1223–1230
Nagasawa H, Little JB (1992) Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res 52:6394–6396
Littlefield LG, Hollowell JG, Pool WH (1969) Chromosomal aberrations induced by plasma from irradiated patients: an indirect effect of x-irradiation. Radiology 93:879–886
Prise KM, Belyakov OV, Folkard M, Michael BD (1998) Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int J Radiat Biol 74:793–798
Kashino G, Prise KM, Schettino G, Folkard M, Vojnovic B, Michael BD, Suzuki K, Kodama S, Watanabe M (2004) Evidence for induction of DNA double strand breaks in the bystander response to targeted soft X-rays in CHO cells. Mutat Res 556:209–215
Nagasawa H, Little JB (1999) Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle irradiation: evidence for a bystander effect. Radiat Res 152:552–557
Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SI, Wright EG (1992) Transmission of chromosomal instability after plutonium alpha-particle irradiation. Nature 355:738–740
Sawant S, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ (2001) The bystander effect in radiation oncogenesis. I. Transformation can be initiated in the unirradiated neighbours of irradiated cells. Radiat Res 155:397–401
Mitchell SA, Marino SA, Brenner DJ, Hall EJ (2004) Bystander effect and adaptive response in C3H10T1/2 cells. Int J Radiat Biol 80:465–472
Hickman AW, Jaramillo RJ, Lechner JF, Johnson NF (1994) Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer Res 54:5797–5800
Iyer R, Lehnert BE (2000) Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res 60:1290–1298
Narayanan PK, Goodwin EH, Lehnert BE (1997) Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 57:3963–3971
Azzam EI, de Toledo SM, Spitz DR, Little JB (2002) Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res 62:5436–5442
Iyer R, Lehnert BE (2002) Alpha-particle-induced increase in the radioresistance of normal human bystander cells. Radiat Res 157:3–7
Iyer R, Lehnert BE (2002) Low dose, low LET ionizing radiation-induced radioadaptation and associated early response in unirradiated cells. Mutat Res 503:1–9
Nagasawa H, Huo L, Little JB (2003) Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int J Radiat Biol 79:35–41
Little JB, Nagasawa H, Li GC, Chen DJ (2003) Involvement of the nonhomologous end joining DNA repair pathway in the bystander effect for chromosomal aberrations. Radiat Res 159:262–267
Mothersill C, Seymour RJ, Seymour CB (2004) Bystander effects in repair-deficient cell lines. Radiat Res 161:256–263
Wang B, Ohyama H, Shang Y, Fujita K, Tanaka K, Nakajima T, Aizawa S, Yukawa O, Hayata I (2004) Adaptive response in embryogenesis: IV. Protective and detrimental bystander effects induced by X radiation in cultured limb bud cells of foetal mice. Radiat Res 161:9–16
Azzam EI, de Toledo SM, Little JB (2001) Direct evidence for the participation of gap-junction mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to non-irradiated cells. Proc Natl Acad Sci USA 98:473–478
Shao C, Furusawa Y, Aoki M, Ando K (2003) Role of gap junctional intercellular communication in radiation-induced bystander effects in human fibroblasts. Radiat Res 160:318–323
Mitchell SA, Randers-Pehrson G, Brenner DJ, Hall EJ (2004) The bystander response in C3H10T1/2 cells: the influence of cell-to-cell contact. Radiat Res 161:397–401
Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K (2002) Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumor cells. Int J Radiat Biol 78:837–844
Azzam EI, de Toledo SM, Gooding T, Little JB (1998) Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res 150:497–504
Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, Waldren CA, Hei TK (2001) Radiation risk to low fluences of a particles may be greater than we thought. Proc Natl Acad Sci USA 98:14410–14415
Nagasawa H, Cremesti A, Kolesnick R, Fuks Z, Little JB (2002) Involvement of membrane signalling in the bystander effect in irradiated cells. Cancer Res 62:2531–2534
Whitmore GF, Varghese GAJ, Gulyas S (1989) Cell cycle-responses of two X-ray sensitive mutants defective in DNA-repair. Int J Radiat Biol 56:657–665
Fuller LF, Painter RB (1988) A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat Res 193:109–121
Greif KD, Brede HJ, Frankenberg D, Giesen U (2004) The PTB single ion microbeam for irradiation of living cells. Nucl Instrum Methods Phys Res B 217:505–512
Greif K, Beverung W, Langner F, Frankenberg D, Gellhaus A, Banaz-Yasar F (2006) The PTB microbeam: a versatile instrument for radiobiological research. Radiat Prot Dosimetry 122:313–315
Hinz JM, Yamada NA, Salazar EP, Tebbs RS, Thompson LH (2005) Influence of double-strand break repair pathways on radiosensitivity throughout the cell cycle in CHO cells. DNA Repair 4:782–792
Frankenberg-Schwager M, Gebauer A, Koppe C, Wolf S, Pralle E, Frankenberg D (submitted) The role of nonhomologous end joining, conservative homologous recombination and single-strand annealing in the cell cycle dependent repair of DNA double-strand breaks induced by sparsely or densely ionising radiation in mammalian cells. Radiat Res
Pastink A, Eeken JCJ, Lohman PHM (2001) Genomic integrity and the repair of double strand DNA breaks. Mutat Res 480–481:37–50
Valerie K, Povirk LF (2003) Regulation and mechanisms of mammalian double-strand break repair. Oncogene 22:5792–5812
Jeggo PA (1997) DNA-PK: at the cross-roads of biochemistry and genetics. Mutat Res 384:1–14
Lieber ME, Ma YM, Pannicke U, Schwarz K (2003) Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol 4:712–720
Rothkamm K, Krüger I, Thompson LH, Löbrich M (2003) Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 23:5706–5715
Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM (2007) ATR-dependent radiation-induced γH2AX foci in bystander primary human astrocytes and glioma cells. Oncogene 26:993–1002
Van Buul PPW, van Duijn-Goedhart A (submitted) Effects of DNA double-strand breaks repair pathways on the production of radiogenic chromosomal aberrations throughout the cell cycle in V79 Chinese hamster cells
Buxton GW (1987) Radiation chemistry of the liquid state. (1) Water and homogeneous aqueous solutions. In: Farhataziz A, Rodgers MAJ (eds) Radiation chemistry. Wiley, New York, pp 321–350
Nagasawa H, Peng Y, Wilson PF, Lio Y-C, Chen DJ, Bedford JS, Little JB (2005) Role of homologous recombination in the alpha-particle-induced bystander effect for sister chromatid exchanges and chromosomal aberrations. Radiat Res 164:141–147
Nagasawa H, Little JB (2002) Bystander effect for chromosomal aberrations induced in wild-type and repair deficient CHO cells by low fluences of alpha particles. Mutat Res 508:121–129
Hashimoto M, Donald CD, Yannone SM, Chen DJ, Roy R, Kow YW (2001) A possible role of Ku in mediating sequential repair of closely opposed lesions. J Biol Chem 276:12827–12831
International Organization for Standardization (ISO) (1995) Guide to the expression of uncertainty in measurement. ISO, Geneva
Acknowledgements
This work was supported by a grant from the European Commission (FIGH-CT-2002-00218). The authors would like to thank O. Döhr, H. Eggestein, T. Heldt and M. Hoffmann for the operation of the PTB cyclotron, K. Beverung, A. Heiske and S. Löb for their constant support with the irradiation of cells and E. Eggestein and B. Heise for their support in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frankenberg, D., Greif, KD., Beverung, W. et al. The role of nonhomologous end joining and homologous recombination in the clonogenic bystander effects of mammalian cells after exposure to counted 10 MeV protons and 4.5 MeV α-particles of the PTB microbeam. Radiat Environ Biophys 47, 431–438 (2008). https://doi.org/10.1007/s00411-008-0187-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-008-0187-7