Abstract
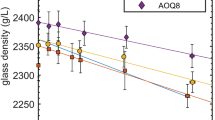
The molar volumes of 19 hydrous albitic liquids (1.9 to 6.1 wt% H2Ototal) were determined at one bar and 505–765 K. These volume data were derived from density measurements on hydrous glasses at 298 K, followed by measurements of the thermal expansion of each glass from 298 K to its respective glass transition temperature. The technique exploits the fact that the volume of a glass is equal to that of the corresponding liquid at the limiting fictive temperature (T f′), and that T f′ can be approximated as the temperature near the onset of the rapid increase in thermal expansion that occurs in the glass transition interval. The volume data of this study were combined with available volume data for anhydrous, Na2O-Al2O3-SiO2 liquids to derive the partial molar volume (±1) of the H2O component in an albitic melt at ∼565 K and one bar. To extend the determination of to higher temperatures and pressures, the molar volumes of the hydrous albitic liquids determined in this study were combined with those measured by previous authors at 1023–1223 K and 480–840 MPa, leading to the following fitted values (±1) at 1673 K and one bar: (±0.46)×10−3 cm−3/mol-K, and dV¯ H 2 O total/dP=−3.82 (±0.36)×10−4 cm3/mol-bar. The measured molar volumes of this study and those of previous authors can be recovered with a standard deviation of 0.5%, which is within the respective experimental errors. There is a significant difference between the values for derived in this study as a function of temperature and pressure and those obtained from an existing polynomial, primarily caused by the previous absence of accurate density measurements on anhydrous silicate liquids. The coefficients of thermal expansion (=4.72×10−4/K) and isothermal compressibility ( T =1.66×10−5/bar) for the H2O component at 1273 K and 100 MPa, indicate that H2Ototal is the single most expansive and compressible component in silicate liquids. For example, at 1473 K and 70 MPa (conditions of a mid-ocean ridge crustal magma chamber), the presence of just 0.4 wt% H2O will decrease the density of a basaltic liquid by more than one percent. An equivalent decrease in melt density could be achieved by increasing the temperature by 175 degrees or the decreasing pressure by 230 MPa. Therefore, even minor quantities of dissolved water will have a marked effect on the dynamic properties of silicate liquids in the crustal environment.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 20 August 1996 / Accepted: 15 March 1997
Rights and permissions
About this article
Cite this article
Ochs III., F., Lange, R. The partial molar volume, thermal expansivity, and compressibility of H2O in NaAlSi3O8 liquid: new measurements and an internally consistent model. Contrib Mineral Petrol 129, 155–165 (1997). https://doi.org/10.1007/s004100050329
Issue Date:
DOI: https://doi.org/10.1007/s004100050329