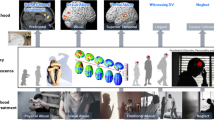
Abstract
Infections during pregnancy are associated with an increased risk of neuropsychiatric disorders with developmental etiologies, such as schizophrenia and autism spectrum disorders (ASD). Studies have shown that the animal model of maternal immune activation (MIA) reproduces a wide range of phenotypes relevant to the study of neurodevelopmental disorders. Emerging evidence shows that (R)-ketamine attenuates behavioral, cellular, and molecular changes observed in animal models of neuropsychiatric disorders. Here, we investigate whether (R)-ketamine administration during adolescence attenuates some of the phenotypes related to neurodevelopmental disorders in an animal model of MIA. For MIA, pregnant Swiss mice received intraperitoneally (i.p.) lipopolysaccharide (LPS; 100 µg/kg/day) or saline on gestational days 15 and 16. The two MIA-based groups of male offspring received (R)-ketamine (20 mg/kg/day; i.p.) or saline from postnatal day (PND) 36 to 50. At PND 62, the animals were examined for anxiety-like behavior and locomotor activity in the open-field test (OFT), as well as in the social interaction test (SIT). At PND 63, the prefrontal cortex (PFC) was collected for analysis of oxidative balance and gene expression of the cytokines IL-1β, IL-6, and TGF-β1. We show that (R)-ketamine abolishes anxiety-related behavior and social interaction deficits induced by MIA. Additionally, (R)-ketamine attenuated the increase in lipid peroxidation and the cytokines in the PFC of the offspring exposed to MIA. The present work suggests that (R)-ketamine administration may have a long-lasting attenuation in deficits in emotional behavior induced by MIA, and that these effects may be attributed to its antioxidant and anti-inflammatory activity in the PFC.





Similar content being viewed by others
Data availability
Not applicable.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Ago Y, Tanabe W, Higuchi M, Tsukada S, Tanaka T, Yamaguchi T, Igarashi H, Yokoyama R, Seiriki K, Kasai A, Nakazawa T, Nakagawa S, Hashimoto K, Hashimoto H (2019) (r)-ketamine induces a greater increase in prefrontal 5-ht release than (s)-ketamine and ketamine metabolites via an ampa receptor-independent mechanism. Int J Neuropsychopharmacol 22:665–674. https://doi.org/10.1093/ijnp/pyz041
Baharnoori M, Bhardwaj SK, Srivastava LK (2012) Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: A prenatal infection model for developmental neuropsychiatric disorders. Schizophr Bull 38:444–456. https://doi.org/10.1093/schbul/sbq098
Bello-Arroyo E, Roque H, Marcos A, Orihuel J, Higuera-Matas A, Desco M, Caiolfa VR, Ambrosio E, Lara-Pezzi E, Gómez-Gaviro MV (2018) Moubeat: A new and open toolbox for guided analysis of behavioral tests in mice. Front Behav Neurosci 12:201. https://doi.org/10.3389/fnbeh.2018.00201
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brown AS, Meyer U (2018) Maternal immune activation and neuropsychiatric illness: A translational research perspective. Am J Psychiatry 175:1073–1083. https://doi.org/10.1176/appi.ajp.2018.17121311
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/s0076-6879(78)52032-6
Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134:49–57. https://doi.org/10.1016/s0166-4328(01)00452-1
Casquero-Veiga M, García-García D, MacDowell KS, Pérez-Caballero L, Torres-Sánchez S, Fraguas D, Berrocoso E, Leza JC, Arango C, Desco M, Soto-Montenegro ML (2019) Risperidone administered during adolescence induced metabolic, anatomical and inflammatory/oxidative changes in adult brain: A pet and mri study in the maternal immune stimulation animal model. European Neuropsychopharmacol 29:880–896. https://doi.org/10.1016/j.euroneuro.2019.05.002
Cecerska-Heryć E, Polikowska A, Serwin N, Roszak M, Grygorcewicz B, Heryć R, Michalczyk A, Dołęgowska B (2022) Importance of oxidative stress in the pathogenesis, diagnosis, and monitoring of patients with neuropsychiatric disorders, a review. Neurochemistry International 153: 105269, doi: https://doi.org/10.1016/j.neuint.2021.105269
Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, Ren Q, Dong C, Fujita Y, Hashimoto K (2019) Comparison of antidepressant and side effects in mice after intranasal administration of (r, s)-ketamine, (r)-ketamine, and (s)-ketamine. Pharmacol Biochem Behav 181:53–59. https://doi.org/10.1016/j.pbb.2019.04.008
Chen L, Shi XJ, Liu H, Mao X, Gui LN, Wang H, Cheng Y (2021) Oxidative stress marker aberrations in children with autism spectrum disorder: A systematic review and meta-analysis of 87 studies (n = 9109). Transl Psychiatry 11:15. https://doi.org/10.1038/s41398-020-01135-3
Chomczynski P, Sacchi N (1987) Single-step method of rna isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. https://doi.org/10.1006/abio.1987.9999
Cieślik M, Gąssowska-Dobrowolska M, Jęśko H, Czapski GA, Wilkaniec A, Zawadzka A, Dominiak A, Polowy R, Filipkowski RK, Boguszewski PM, Gewartowska M, Frontczak-Baniewicz M, Sun GY, Beversdorf DQ, Adamczyk A (2020) Maternal immune activation induces neuroinflammation and cortical synaptic deficits in the adolescent rat offspring. Int J Mol Sci. https://doi.org/10.3390/ijms21114097
da Silva AI, Braz GRF, Silva SCA, Pedroza A, de Lima-Júnior NC, Silva TLA, Lagranha CJ (2019) Body composition, biochemical, behavioral and molecular alterations in overfed rats after chronic exposure to ssri. Behav Brain Res 356:62–70. https://doi.org/10.1016/j.bbr.2018.08.007
de Theije CG, Wu J, Koelink PJ, Korte-Bouws GA, Borre Y, Kas MJ, Lopes da Silva S, Korte SM, Olivier B, Garssen J, Kraneveld AD (2014) Autistic-like behavioural and neurochemical changes in a mouse model of food allergy. Behav Brain Res 261:265–274. https://doi.org/10.1016/j.bbr.2013.12.008
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Estes ML, McAllister AK (2016) Maternal immune activation: Implications for neuropsychiatric disorders. Science (New York, NY) 353:772–777. https://doi.org/10.1126/science.aag3194
Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM (2016) Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321:197–209. https://doi.org/10.1016/j.neuroscience.2015.07.041
Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, Leza JC, Arango C (2019) Oxidative stress and inflammation in first-episode psychosis: A systematic review and meta-analysis. Schizophr Bull 45:742–751. https://doi.org/10.1093/schbul/sby125
Frohlich J, Van Horn JD (2014) Reviewing the ketamine model for schizophrenia. J Psychopharmacol (Oxford, England) 28:287–302. https://doi.org/10.1177/0269881113512909
Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013) Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68. https://doi.org/10.1016/j.bbi.2012.07.008
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione s-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Han VX, Patel S, Jones HF, Dale RC (2021) Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol 17:564–579. https://doi.org/10.1038/s41582-021-00530-8
Hashimoto K (2023) Arketamine for cognitive impairment in psychiatric disorders. European Arch Psychiatry Clin Neurosci, doi: https://doi.org/10.1007/s00406-023-01570-5
Jiang NM, Cowan M, Moonah SN, Petri WA Jr (2018) The impact of systemic inflammation on neurodevelopment. Trends Mol Med 24:794–804. https://doi.org/10.1016/j.molmed.2018.06.008
Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, O’Donovan M, Correll CU, Kane JM, van Os J, Insel TR (2015) Schizophrenia Nat Rev Dis Primers 1:15067. https://doi.org/10.1038/nrdp.2015.67
Kerns CM, Kendall PC (2012) The presentation and classification of anxiety in autism spectrum disorder, Clin Physchol 19: 323–347, doi: https://doi.org/10.1111/cpsp.12009
Lai MC, Lombardo MV, Baron-Cohen S (2014) Autism. Lancet (London, England) 383:896–910. https://doi.org/10.1016/s0140-6736(13)61539-1
Lanté F, Meunier J, Guiramand J, De Jesus Ferreira MC, Cambonie G, Aimar R, Cohen-Solal C, Maurice T, Vignes M, Barbanel G (2008) Late n-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus 18:602–609. https://doi.org/10.1002/hipo.20421
Le Merre P, Ährlund-Richter S, Carlén M (2021) The mouse prefrontal cortex: Unity in diversity. Neuron 109:1925–1944. https://doi.org/10.1016/j.neuron.2021.03.035
Leal GC, Souza-Marques B, Mello RP, Bandeira ID, Caliman-Fontes AT, Carneiro BA, Faria-Guimarães D, Guerreiro-Costa LNF, Jesus-Nunes AP, Silva SS, Lins-Silva DH, Fontes MA, Alves-Pereira R, Cordeiro V, Rugieri-Pacheco S, Santos-Lima C, Correia-Melo FS, Vieira F, Sanacora G, Lacerda ALT, Quarantini LC (2023) Arketamine as adjunctive therapy for treatment-resistant depression: A placebo-controlled pilot study. J Affect Disord 330:7–15. https://doi.org/10.1016/j.jad.2023.02.151
Lee GA, Lin YK, Lai JH, Lo YC, Yang YSH, Ye SY, Lee CJ, Wang CC, Chiang YH, Tseng SH (2021) Maternal immune activation causes social behavior deficits and hypomyelination in male rat offspring with an autism-like microbiota profile. Brain Sci. https://doi.org/10.3390/brainsci11081085
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods (San Diego, Calif) 25:402–408. https://doi.org/10.1006/meth.2001.1262
Ma L, Wang L, Chang L, Shan J, Qu Y, Wang X, Fujita Y, Hashimoto K (2022) A role of microrna-149 in the prefrontal cortex for prophylactic actions of (r)-ketamine in inflammation model. Neuropharmacology 219:109250. https://doi.org/10.1016/j.neuropharm.2022.109250
MacDowell KS, Munarriz-Cuezva E, Caso JR, Madrigal JL, Zabala A, Meana JJ, García-Bueno B, Leza JC (2017) Paliperidone reverts toll-like receptor 3 signaling pathway activation and cognitive deficits in a maternal immune activation mouse model of schizophrenia. Neuropharmacology 116:196–207. https://doi.org/10.1016/j.neuropharm.2016.12.025
Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ (2015) Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol Psychiatry 20:440–446. https://doi.org/10.1038/mp.2014.59
Massrali A, Adhya D, Srivastava DP, Baron-Cohen S, Kotter MR (2022) Virus-induced maternal immune activation as an environmental factor in the etiology of autism and schizophrenia. Front Neurosci 16:834058. https://doi.org/10.3389/fnins.2022.834058
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Murray CA, Clements MP, Lynch MA (1999) Interleukin-1 induces lipid peroxidation and membrane changes in rat hippocampus: An age-related study. Gerontology 45:136–142. https://doi.org/10.1159/000022076
Parise EM, Alcantara LF, Warren BL, Wright KN, Hadad R, Sial OK, Kroeck KG, Iñiguez SD, Bolaños-Guzmán CA (2013) Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol Psychiat 74:750–759. https://doi.org/10.1016/j.biopsych.2013.04.027
Radtke FA, Chapman G, Hall J, Syed YA (2017) Modulating neuroinflammation to treat neuropsychiatric disorders. Biomed Res Int 2017:5071786. https://doi.org/10.1155/2017/5071786
Reznick AZ, Packer L (1994) Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363. https://doi.org/10.1016/s0076-6879(94)33041-7
Scotton E, Antqueviezc B, Vasconcelos MF, Dalpiaz G, Paul Géa L, Ferraz Goularte J, Colombo R, Ribeiro Rosa A (2022) Is (r)-ketamine a potential therapeutic agent for treatment-resistant depression with less detrimental side effects? A review of molecular mechanisms underlying ketamine and its enantiomers. Biochem Pharmacol 198:114963. https://doi.org/10.1016/j.bcp.2022.114963
Sies H (2020) Oxidative stress: Concept and some practical aspects. Antioxidants. https://doi.org/10.3390/antiox9090852
Simões LR, Sangiogo G, Tashiro MH, Generoso JS, Faller CJ, Dominguini D, Mastella GA, Scaini G, Giridharan VV, Michels M, Florentino D, Petronilho F, Réus GZ, Dal-Pizzol F, Zugno AI, Barichello T (2018) Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J Psychiatr Res 100:71–83. https://doi.org/10.1016/j.jpsychires.2018.02.007
Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES (2018) Maternal immune activation in neurodevelopmental disorders. Develop Dynam 247:588–619. https://doi.org/10.1002/dvdy.24612
Talukdar PM, Abdul F, Maes M, Binu VS, Venkatasubramanian G, Kutty BM, Debnath M (2020) Maternal immune activation causes schizophrenia-like behaviors in the offspring through activation of immune-inflammatory, oxidative and apoptotic pathways, and lowered antioxidant defenses and neuroprotection. Mol Neurobiol 57:4345–4361. https://doi.org/10.1007/s12035-020-02028-8
Tan Y, Fujita Y, Pu Y, Chang L, Qu Y, Wang X, Hashimoto K (2022) Repeated intermittent administration of (r)-ketamine during juvenile and adolescent stages prevents schizophrenia-relevant phenotypes in adult offspring after maternal immune activation: A role of trkb signaling. Eur Arch Psychiatry Clin Neurosci 272:693–701. https://doi.org/10.1007/s00406-021-01365-6
Tartaglione AM, Villani A, Ajmone-Cat MA, Minghetti L, Ricceri L, Pazienza V, De Simone R, Calamandrei G (2022) Maternal immune activation induces autism-like changes in behavior, neuroinflammatory profile and gut microbiota in mouse offspring of both sexes. Transl Psychiatry 12:384. https://doi.org/10.1038/s41398-022-02149-9
Temme L, Schepmann D, Schreiber JA, Frehland B, Wünsch B (2018) Comparative pharmacological study of common nmda receptor open channel blockers regarding their affinity and functional activity toward glun2a and glun2b nmda receptors. ChemMedChem 13:446–452. https://doi.org/10.1002/cmdc.201700810
Temmingh H, Stein DJ (2015) Anxiety in patients with schizophrenia: Epidemiology and management. CNS Drugs 29:819–832. https://doi.org/10.1007/s40263-015-0282-7
Wei Y, Chang L, Hashimoto K (2022) Molecular mechanisms underlying the antidepressant actions of arketamine: Beyond the nmda receptor. Mol Psychiatry 27:559–573. https://doi.org/10.1038/s41380-021-01121-1
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Translational psychiatry 5:e632. https://doi.org/10.1038/tp.2015.136
Zanos P, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, Morris PJ, Stewart BW, Thomas CJ, Thompson SM, Moaddel R, Gould TD (2019) (r)-ketamine exerts antidepressant actions partly via conversion to (2r,6r)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br J Pharmacol 176:2573–2592. https://doi.org/10.1111/bph.14683
Zhang J, Ma L, Wan X, Shan J, Qu Y, Hashimoto K (2021) (r)-ketamine attenuates lps-induced endotoxin-derived delirium through inhibition of neuroinflammation. Psychopharmacology 238:2743–2753. https://doi.org/10.1007/s00213-021-05889-6
Zhang JC, Li SX, Hashimoto K (2014) R (-)-ketamine shows greater potency and longer lasting antidepressant effects than s (+)-ketamine. Pharmacol Biochem Behav 116:137–141. https://doi.org/10.1016/j.pbb.2013.11.033
Zuckerman L, Rehavi M, Nachman R, Weiner I (2003) Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: A novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 28:1778–1789. https://doi.org/10.1038/sj.npp.1300248
Acknowledgements
The authors are grateful for the fellowship granted to Elifrances Galdino de Oliveira by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001) and the Science and Technology Support Foundation of the State of Pernambuco (FACEPE, process BCT-0327-4.01/21).
Funding
There was no financial support for the development of this research.
Author information
Authors and Affiliations
Contributions
EGO: conceptualization, data curation, formal analysis, investigation, methodology, roles/writing—original draft, writing—review & editing. DAL: investigation, methodology. JCSJ: investigation, methodology. MVSB: investigation, methodology. SCAS: methodology, data curation, investigation. JHS: methodology, data curation, investigation. OHSJ: methodology, data curation, investigation. ECL: validation, visualization, funding acquisition, resources. CJL: conceptualization, methodology, data curation, investigation, writing—review & editing. FSD: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, roles/writing—original draft, writing—review & editing. DAG: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, roles/writing—original draft, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, E.G., de Lima, D.A., da Silva Júnior, J.C. et al. (R)-ketamine attenuates neurodevelopmental disease-related phenotypes in a mouse model of maternal immune activation. Eur Arch Psychiatry Clin Neurosci 273, 1501–1512 (2023). https://doi.org/10.1007/s00406-023-01629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-023-01629-3