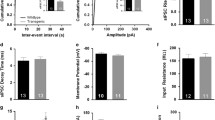
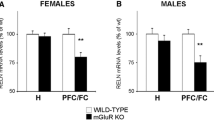
Abstract
The molecular etiology of schizophrenia comprises abnormal neurotransmission of the amino acid GABA (γ-aminobutyric acid). Neuropathological studies convincingly revealed reduced expression of glutamic acid decarboxylase (Gad67) in GABAergic interneurons. Several antipsychotics influence the expression of GABAergic genes, but aripiprazole (APZ), a partial dopaminergic and serotonergic receptor agonist, has not been involved into these studies so far. We treated Sprague–Dawley rats for 4 weeks or 4 months with APZ suspended in drinking water and doses of 10 and 40 mg per kg body weight. Gene expression of Gad67, the vesicular GABA transporter Slc32a1 (solute carrier family, Vgat), the transmembrane transporters Slc6a1 (Gat1) and Slc6a11 (Gat3) was assessed by semiquantitative radioactive in situ hybridization. APZ treatment resulted in time- and dose-dependent effects with qualitative differences between brain regions. In the 10-mg group, Slc6a1 was strongly induced after 4 weeks in the hippocampus, amygdala, and cerebral cortex, followed by an induction of Gad67 in the same regions after 4 months, while frontocortical regions as well as basal ganglia showed dose-dependent reductions of Gad67 expression after 4 months. In several frontocortical and subcortical regions, we observed a decrease of Slc32a1 and an increase of Slc6a11 expression. In conclusion, APZ modulates gene expression of GABAergic marker genes involved into pathogenetic theories of schizophrenia. APZ only partially mirrors the effects of other antipsychotics with some important differences regarding brain regions. The findings might be explained by regulatory connections between serotonergic, GABAergic, and dopaminergic neurotransmission and should be validated in behavioral animal models of psychotic disorders.





Similar content being viewed by others
Abbreviations
- ACC:
-
Anterior cingulate cortex
- AM:
-
Amygdala
- APZ:
-
Aripiprazole
- CA1:
-
Hippocampal subregion cornu ammonis 1
- CA3:
-
Hippocampal subregion cornu ammonis 3
- CPU:
-
Caudate nucleus and putamen
- DG:
-
Granular layer of dentate gyrus
- EAAT:
-
Excitatory amino acid transporter
- FPC:
-
Frontal part of parietal cortex
- GABA:
-
γ-Aminobutyric acid
- GAT:
-
GABA transporter
- HB:
-
Habenula
- Hypoth:
-
Hypothalamic nuclei
- ISH:
-
In situ hybridization
- Limb:
-
Nuclei of horizontal and vertical limbic diagonal band
- mRNA:
-
Messenger ribonucleic acid
- NMDA:
-
N-methyl-d-aspartate
- NR:
-
NMDA-receptor
- NT:
-
Nucleotides
- OC:
-
Occipital cortex
- PC:
-
Parietal cortex (dorsal part)
- PFC:
-
Prefrontal cortex
- RSG:
-
Retrosplenial granular cortex
- SEM:
-
Standard error of the mean
- TC:
-
Temporal cortex
- TH:
-
Thalamus
- Slc32a1:
-
Vesicular GABA transporter
References
Rolls ET, Loh M, Deco G, Winterer G (2008) Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci 9:696–709
Benes FM (2009) Neural circuitry models of schizophrenia: is it dopamine, GABA, glutamate, or something else? Biol Psychiatry 65:1003–1005
Lopez-Gil X, Artigas F, Adell A (2010) Unraveling monoamine receptors involved in the action of typical and atypical antipsychotics on glutamatergic and serotonergic transmission in prefrontal cortex. Curr Pharmaceut Design 16:502–515
Meltzer HY, Fonnum F (1987) Biochemistry, anatomy, and pharmacology of GABA neurons. Psychopharmacol 18:173–182
Hendry SH, Jones EG, Emson PC, Lawson DE, Heizmann CW, Streit P (1989) Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res 76:467–472
Gadea A, Lopez-Colome AM (2001) Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J Neurosci Res 63:461–468
Gonzalez-Burgos G, Rotaru DC, Zaitsev AV, Povysheva NV, Lewis DA (2009) GABA transporter GAT1 prevents spillover at proximal and distal GABA synapses onto primate prefrontal cortex neurons. J Neurophysiol 101:533–547
Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH et al (1998) The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 18:9733–9750
Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacol 25:1–27
Lewis DA, Hashimoto T, Volk DW (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324
Schmidt MJ, Mirnics K (2012) Modeling interneuron dysfunction in schizophrenia. Dev Neurosci. doi:10.1159/000336731
Gonzalez-Burgos G, Hashimoto T, Lewis DA (2010) Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 12:335–344
Lewis DA (2009) Neuroplasticity of excitatory and inhibitory cortical circuits in schizophrenia. Dial Clin Neurosci 11:269–280
Volman V, Behrens MM, Sejnowski TJ (2011) Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 31:18137–18148
Bitanihirwe BK, Lim MP, Kelley JF, Kaneko T, Woo TU (2009) Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry 9:71
Romon T, Mengod G, Adell A (2011) Expression of parvalbumin and glutamic acid decarboxylase-67 after acute administration of MK-801. Implications for the NMDA hypofunction model of schizophrenia. Psychopharmacol 217:231–238
Braun I, Genius J, Grunze H, Bender A, Möller HJ, Rujescu D (2007) Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr Res 97:254–263
Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H et al (2006) A pharmacological model for psychosis based on N-methyl-d-aspartate receptor hypofunction: molecular, cellular, functional and behaviorial abnormalities. Biol Psychiatry 59:721–729
Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y et al (2010) Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13:76–83
Fitzgerald PB (2010) BL-1020, an oral antipsychotic agent that reduces dopamine activity and enhances GABAA activity, for the treatment of schizophrenia. Curr Opin Invest Drugs 11:92–100
Stan A, Lewis D (2012) Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr Pharmac Biotechnol 13:1557–1562
Ibrahim HM, Tamminga CA (2012) Treating impaired cognition in schizophrenia. Curr Pharmac Biotechnol 13:1587–1594
Genius J, Giegling I, Benninghoff J, Rujescu D (2012) Disturbed function of GABAergic interneurons in schizophrenia: relevance for medical treatment? Curr Pharmac Biotechnol 13:1549–1556
Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA (2008) Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry 165:479–489
Curley AA, Arion D, Volk DW, Asufa-Adjei JK, Sampson AR, Fish KN et al (2012) Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry 168:921–929
Fung SJ, Webster MJ, Weicker CS (2011) Expression of VGluT1 and VGAT mRNAs in human dorsolateral prefrontal cortex during development and in schizophrenia. Brain Res 1388:22–31
Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA (2009) Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharmacol 34:2112–2124
Reynolds GP, Czudek C, Andrews HB (1990) Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biol Psychiatry 27:1038–1044
Benes FM, Baretta S (2000) Amygdalo-entorhinal inputs to the hippocampla formation in relation to schizophrenia. Ann NY Acad Sci 911:293–304
Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S et al (2010) GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res 117:83–91
Perry TL, Hansen S, Kish SJ (1979) Effects of chronic administration of antipsychotic drugs on GABA and other amino acids in the mesolimbic area of rat brain. Life Sci 24:283–288
Hashimoto T, Arion D, Unger T, Maldonado A, Morris HM, Volk DW et al (2008) Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 13:147–161
Gunne LM, Haggstrom JE, Sjoquist B (1984) Association with persistent neuroleptic-induced dyskinesia of regional changes in brain GABA synthesis. Nature 309:347–349
Delfs JM, Ellison GD, Mercugliano M, Chesselet MF (1995) Expression of glutamic acid decarboxylase mRNA in striatum and pallidum in an animal model of tardive dyskinesia. Exp Neurol 133:175–188
Jolkkonen J, Jenner P, Marsden CD (1994) GABAergic modulation of striatal peptide expression in rats and the alterations induced by dopamine antagonist treatment. Neurosci Lett 180:273–276
Delfs JM, Anegawa NJ, Chesselet MF (1995) Glutamate decarboxylase messenger RNA in rat pallidum: comparison of the effects of haloperidol, clozapine and combined haloperidol-scopolamine treatments. Neurosci 66:67–80
Laprade N, Soghomonian JJ (1995) Differential regulation of mRNA levels encoding for the two isoforms of glutamate decarboxylase (GAD65 and GAD67) by dopamine receptors in the rat striatum. Mol Brain Res 34:65–74
Sakai K, Gao XM, Hashimoto T, Tamminga CA (2001) Traditional and new antipsychotic drugs differentially alter neurotransmission markers in basal ganglia-thalamocortical neural pathways. Synapse 39:152–160
Zink M, Schmitt A, May B, Müller B, Demirakca T, Braus DF et al (2004) Differential effects of long-term treatment with clozapine of haloperidol on GABAa receptor binding and GAD67 expression. Schizophr Res 66:151–157
Chen JF, Weiss B (1993) Irreversible blockade of D2 dopamine receptors by fluphenazine-N- mustard increases glutamic acid decarboxylase mRNA in rat striatum. Neurosci Lett 150:215–218
Johnson AE, Liminga U, Liden A, Lindefors N, Gunne LM, Wiesel FA (1994) Chronic treatment with a classical neuroleptic alters excitatory amino acid and GABAergic neurotransmission in specific regions of the rat brain. Neurosci 63:1003–1020
Daskalakis ZJ, George TP (2009) Clozapine, GABA(B), and the treatment of resistant schizophrenia. Clin Pharmacol Therap 86:442–446
Farnbach-Pralong D, Bradbury R, Copolov D, Dean B (1998) Clozapine and olanzapine treatment decreases rat cortical and limbic GABA(A) receptors. Eur J Pharmacol 349:R7–R8
Amitai N, Kuczenski R, Behrens MM, Markou A (2012) Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats. Neuropharmacol 62:1422–1431
Zink M, Schmitt A, May B, Müller B, Braus DF, Henn FA (2004) Differential effects of long-term treatment with clozapine or haloperidol on GABA-transporter expression. Pharmacopsychiatry 37:171–174
Yamamura S, Ohoyama K, Hamaguchi T, Kashimoto K, Nakagawa M, Kanehara S et al (2009) Effects of quetiapine on monoamine, GABA, and glutamate release in rat prefrontal cortex. Psychopharmacol 206:243–258
Ohoyama K, Yamamura S, Hamaguchi T, Nakagawa M, Motomura E, Shiroyama T et al (2011) Effect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortex. Eur J Pharmacol 653:47–57
Yamamura S, Ohoyama K, Hamaguchi T, Nakagawa M, Suzuki D, Matsumoto T et al (2009) Effects of zotepine on extracellular levels of monoamine, GABA and glutamate in rat prefrontal cortex. Br J Pharmacol 157:656–665
Goto N, Yoshimura R, Kakeda S, Moriya J, Hayashi K, Ikenouchi-Sugita A et al (2009) Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Hum Psychopharmacol 24:639–645
Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A et al (2009) Reduction of brain [gamma]-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res 112:192–193
Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X et al (2012) Elevated prefrontal cortex gamma¦-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69:449–459
Natesan S, Reckless GE, Barlow KBL, Nobrega JN, Kapur S (2011) Partial agonists in schizophrenia—why some work and others do not: insights from preclinical animal models. Int J Neuropsychopharmacol 14:1165–1178
Bortolozzi A, az-Mataix L, Toth M, Celada P, Artigas F (2007) In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacol 191:745–758
Sparshatt A, Taylor D, Patel MX, Kapur S (2010) A systematic review of aripiprazole–dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoring. J Clin Psychiatry 71:1447–1456
Kern RS, Green MF, Cornblatt BA, Owen JR, McQuade RD, Carson WH et al (2006) The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacol 187:312–320
Hamamura T, Harada T (2007) Unique pharmacological profile of aripiprazole as the phasic component buster. [erratum appears in Psychopharmacology (Berl). 2007 Apr; 191(3):855]. Psychopharmacol 191:741–743
Han M, Huang XF, Deng C (2009) Aripiprazole differentially affects mesolimbic and nigrostriatal dopaminergic transmission: implications for long-term drug efficacy and low extrapyramidal side-effects. Int J Neuropsychopharmacol. doi:10.1017/s1461145709009948
Koprivica V, Regardie K, Wolff C, Fernalld R, Murphy JJ, Kambayashi J et al (2011) Aripiprazole protects cortical neurons from glutamate toxicity. Eur J Pharmacol 651:73–76
Cheng MC, Liao D-L, Hsiung C-A, Chen C-Y, Liao Y-C, Chen C-H (2008) Chronic treatment with aripiprazole induces differential gene expression in the rat frontal cortex. Int J Neuropsychopharmacol 11:207–216
Segnitz N, Schmitt A, Gebicke-Härter P, Zink M (2009) Differential expression of glutamate transporter genes after chronic oral treatment with aripiprazole in rats. Neurochem Int 55:619–628
Segnitz N, Ferbert T, Schmitt A, Gass P, Gebicke-Härter P, Zink M (2011) Effects of chronic oral treatment with aripiprazole on the expression of NMDA receptor subunits and binding sites in rat brain. Psychopharmacol 217:127–142
Llado-Pelfort L, Santana N, Ghisi V, Artigas F, Celada P (2012) 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex 22:1487–1497
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press inc, San Diego
Zink M, Vollmayr B, Gebicke-Härter P, Henn FA (2009) Reduced expression of GABA transporter GAT3 in helpless rats, an animal model of depression. Neurochem Res 34:1584–1593
Van Eden CG, Rinkens A, Uylings HB (1998) Retrograde degeneration of thalamic neurons in the mediodorsal nucleus after neonatal and adult aspiration lesions of the medial prefrontal cortex in the rat. Implications for mechanisms of functional recovery. Eur J Neurosci 10:1581–1589
Van Eden CG, van HA, van HF, Uylings HB (1994) Effects of neonatal mediodorsal thalamic lesions on structure and function of the rat prefrontal cortex. Dev Brain Res 80:26–34
Van Eden CG, Kros JM, Uylings HB (1990) The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Progr Brain Res 85:169–183
Uylings HB, Van Eden CG (1990) Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Progr Brain Res 85:31–62
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, bi-Dargham A et al. (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment: a meta-analysis of imaging studies. Arch Gen Psychiatry 69(8):776–786
Correll CU (2012) Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescent. J Clin Psychiatry 69:26–36
de Almeida J, Mengod G (2008) Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J Neurochemistry 107:488–496
Puig MV, Artigas F, Celada P (2005) Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex 15:1–14
Williams M, Hampton T, Pearce R, Hirsch S, Ansorge O, Thom M et al. (2012) Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-012-0328-50
Schmitt A, Leonardi-Essmann F, Durrenberger P, Wichert S, Spanagel R, Arzberger T et al. (2012) Structural synaptic elements are differentially regulated in superior temporal cortex of schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-012-0306-y
Tamminga CA (2006) The neurobiology of cognition in schizophrenia. J Clin Psychiatry 67:e11
Fitzgerald LW, Deutch AY, Gasic G, Heinemann SF, Nestler EJ (1995) Regulation of cortical and subcortical glutamate receptor subunit expression by antipsychotic drugs. J Neurosci 15:2453–2461
Carli M, Calcagno E, Mainolfi P, Mainini E, Invernizzi RW (2012) Effects of aripiprazole, olanzapine, and haloperidol in a model of cognitive deficit of schizophrenia in rats: relationship with glutamate release in the medial prefrontal cortex. Pharmacopsychiatry 214:639–652
Fell MJ, Katner JS, Rasmussen K, Nikolayev A, Kuo M-S, Nelson DLG et al (2012) Typical and atypical antipsychotic drugs increase extracellular histamine levels in the rat medial prefrontal cortex: contribution of Histamine H1 receptor blockade. Frontiers Psychiatry 3:49. doi:10.3389/fpsyt.2012.00049
Skrede S, Ferno J, Vazquez MJ, Fjaer S, Pavlin T, Lunder N et al (2012) Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. Int J Neuropsychopharmacol 15:163–179
Acknowledgments
This work has been funded by an unrestricted grant by Bristol-Myers Squibb GmbH & CoKGaA and Otsuka Pharmaceuticals to M.Z. The funding source had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Conflict of Interest
M. Z. holds scientific and speaker grants of the European Research Advisory Board (ERAB), German Science Foundation, and Pfizer Pharma GmbH, Servier, Astra Zeneca and Janssen Cilag. N.P., A.S., and P.J. G.-H. have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peselmann, N., Schmitt, A., Gebicke-Haerter, P.J. et al. Aripiprazole differentially regulates the expression of Gad67 and γ-aminobutyric acid transporters in rat brain. Eur Arch Psychiatry Clin Neurosci 263, 285–297 (2013). https://doi.org/10.1007/s00406-012-0367-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-012-0367-y