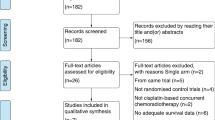
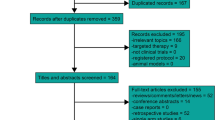
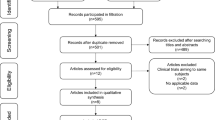
Abstract
Objective
To speculate whether induction chemotherapy (IC) or adjuvant chemotherapy (AC) with concurrent chemoradiotherapy (CCRT) could obtain better survival benefit for stage III or IV locally advanced nasopharyngeal carcinoma (LA-NPC).
Methods
Only randomized controlled trials were incorporated. There were five treatments (CCRT, IC + CCRT, CCRT + AC, IC + RT and RT alone) recruited for analysis. Overall survival (OS), locoregional recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) with a hazard ratio (HR) were selected as endpoints. First of all, we performed a traditional meta-analysis and subsequently conducted network meta-analysis based on the Bayesian method.
Results
Totally, 15 studies, including 6182 patients, were incorporated for analysis. There was a statistically significant benefits in favor of IC + CCRT, compared with CCRT alone, for OS [HR = 0.75, 95% CI = 0.63–0.89], LRFS [HR = 0.70, 95% CI = 0.56–0.86], and DMFS [HR = 0.65, 95% CI = 0.54–0.78]. What's more, we did not observed any significant differences between CCRT + AC and CCRT alone for all the endpoints. Unsurprisingly, it was RT alone that demonstrate the poorest survival benefit. Strange to say, survival benefit, between IC + CCRT and IC + RT, or between IC + CCRT and CCRT + AC, did not significantly exist.
Conclusion
Induction chemotherapy IC + CCRT provided better survival benefit than CCRT alone. CCRT + AC failed to increase survival benefit significantly compared to CCRT alone. More research about comparing IC + CCRT with IC + RT or CCRT + AC are needed.



Similar content being viewed by others
References
Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J (2019) Nasopharyngeal carcinoma. Lancet 394:64–80
Peng L, Liu J, Chen Y, Ma J (2019) The next decade of clinical trials in locoregionally advanced nasopharyngeal carcinoma. British J Radiol 92:20181031
Chen Y, Sun Y, Liang S, Zong J, Li W, Chen M et al (2013) Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer 119:2230–2238
Lee A, Tung S, Ng W, Lee V, Ngan R, Choi H et al (2017) A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer 123:4147–4157
Li W, Chen N, Zhang N, Hu G, Xie F, Sun Y et al (2019) Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer 145:295–305
Lin J, Liang W, Jan J, Jiang R, Lin A (2004) Another way to estimate outcome of advanced nasopharyngeal carcinoma—is concurrent chemoradiotherapy adequate? Int J Radiat Oncol Biol Phys 60:156–164
Colevas A, Yom S, Pfister D, Spencer S, Adelstein D, Adkins D et al (2018) NCCN guidelines insights: head and neck cancers version 1.2018. JNCCN 16:479–490
Zhang Y, Chen L, Hu G-Q, Zhang N, Zhu X-D, Yang K-Y et al (2019) Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 381:1124–1135
Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C et al (2020) Mapping of reporting guidance for systematic reviews and meta-analyses generated a comprehensive item bank for future reporting guidelines. J Clin Epidemiol 118:60–68
Hutton B, Salanti G, Caldwell D, Chaimani A, Schmid C, Cameron C et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Int Med 162:777–784
Woods B, Hawkins N, Scott D (2010) Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol 10:54
Tierney J, Stewart L, Ghersi D, Burdett S, Sydes M (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
Higgins J, Thompson S (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Higgins J, Thompson S, Deeks J, Altman D (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Fryback D, Stout N, Rosenberg M (2001) An elementary introduction to Bayesian computing using WinBUGS. Int J Technol Assess Health Care 17:98–113
Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc 64:583–639
Dias S, Sutton A, Welton N, Ades A (2013) Evidence synthesis for decision making 6: embedding evidence synthesis in probabilistic cost-effectiveness analysis. Med Decis Making 33:671–678
Tan S, Bujkiewicz S, Sutton A, Dequen P, Cooper N (2013) Presentational approaches used in the UK for reporting evidence synthesis using indirect and mixed treatment comparisons. J Health Serv Res Pol 18:224–232
Yang Q, Cao S, Guo L, Hua Y, Huang P, Zhang X et al (2019) Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer 119:87–96
Hong R, Hsiao C, Ting L, Ko J, Wang C, Chang J et al (2018) Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol 29:1972–1979
Cao S, Yang Q, Guo L, Mai H, Mo H, Cao K et al (2017) Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer 75:14–23
Hui E, Ma B, Leung S, King A, Mo F, Kam M et al (2009) Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 27:242–249
Tan T, Lim W, Fong K, Cheah S, Soong Y, Ang M et al (2015) Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 91:952–960
Chen L, Hu C, Chen X, Hu G, Cheng Z, Sun Y et al (2017) Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer 75:150–158
Al-Sarraf M, LeBlanc M, Giri P, Fu K, Cooper J, Vuong T et al (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 16:1310–1317
Wee J, Tan E, Tai B, Wong H, Leong S, Tan T et al (2005) Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 23:6730–6738
Lee AWM, Tung SY, Chan ATC, Chappell R, Fu Y-T, Lu T-X et al (2011) A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol 98:15–22
Huang P, Zeng Q, Cao K, Guo X, Guo L, Mo H et al (2015) Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: a single-institution experience from an endemic area. Europ J Cancer 51:1760–1770
Lee A, Ngan R, Tung S, Cheng A, Kwong D, Lu T et al (2015) Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 121:1328–1338
Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV (> or = N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. Intern J Radiat Oncol Biol Physic. 1996; 35:463–469.
Ma J, Mai H, Hong M, Min H, Mao Z, Cui N et al (2001) Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 19:1350–1357
Sun Y, Li W, Chen N, Zhang N, Hu G, Xie F et al (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17:1509–1520
Chen L, Hu C, Chen X, Hu G, Cheng Z, Sun Y et al (2012) Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 13:163–171
Chen Y, Liu M, Liang S, Zong J, Mao Y, Tang L et al (2008) Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys 71:1356–1364
Lee A, Tung S, Chua D, Ngan R, Chappell R, Tung R et al (2010) Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 102:1188–1198
Lee A, Tung S, Chan A, Chappell R, Fu Y, Lu T et al (2006) Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 66:142–151
Huang P, Cao K, Guo X, Mo H, Guo L, Xiang Y et al (2012) A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol 48:1038–1044
Hong M, Mai H, Min H, Ma J, Zhang E, Cui N (2000) A comparison of the Chinese 1992 and fifth-edition International Union Against Cancer staging systems for staging nasopharyngeal carcinoma. Cancer 89:242–247
Jadad A, Moore R, Carroll D, Jenkinson C, Reynolds D, Gavaghan D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Ribassin-Majed L, Marguet S, Lee A, Ng W, Ma J, Chan A et al (2017) What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol 35:498–505
Bocci G, Kerbel R (2016) Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol 13:659–673
Chen Y, Tang L, Yang Q, Poh S, Hui E, Chan A et al (2018) Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res 24:1824–1833
Tan T, Soon Y, Cheo T, Ho F, Wong L, Tey J et al (2018) Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: a systematic review and meta-analysis. Radiother Oncol 129:10–17
Wang Q, Xu G, Xia Y, Zuo J, Zeng G, Xue Z et al (2020) Comparison of induction chemotherapy plus concurrent chemoradiotherapy and induction chemotherapy plus radiotherapy in locally advanced nasopharyngeal carcinoma. Oral Oncol 111:104925
Chen F, Wen D, Li F, Lin L, Kou J, Zheng W et al (2019) The role of post-neoadjuvant chemotherapy tumor volume for prognostication and treatment guidance in loco-regionally advanced nasopharyngeal carcinoma. Cancers 24:11
Acknowledgement
This study was supported by no fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, L., Shi, L., Wang, W. et al. Which treatment is better than concurrent chemoradiotherapy about survival for stage III or IV locally advanced nasopharyngeal carcinoma? An updated Bayesian network meta-analysis of randomized controlled trials. Eur Arch Otorhinolaryngol 278, 3633–3642 (2021). https://doi.org/10.1007/s00405-021-06614-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06614-x