Abstract
Purpose
The development of a seroma after breast cancer surgery is a common postoperative complication seen after simple mastectomy and axillary surgery. We could recently demonstrate that breast cancer patients undergoing a simple mastectomy with subsequent seroma formation developed a T-helper cell increase within the aspirated fluid measured by flow cytometry. The same study revealed a Th2 and/or a Th17 immune response in peripheral blood and seroma fluid of the same patient. Based on these results and within the same study population, we now analyzed the Th2/Th17 cell associated cytokine content as well as the best known clinical important cytokine IL-6.
Methods
Multiplex cytokine measurements (IL-4, IL-5, IL-13, IL-10, IL-17, and IL-22) were done on 34 seroma fluids (Sf) after fine needle aspiration of patients who developed a seroma after a simple mastectomy. Serum of the same patient (Sp) and that of healthy volunteers (Sc) were used as controls.
Results
We found the Sf to be highly cytokine rich. Almost all analyzed cytokines were significantly higher in abundance in the Sf compared to Sp and Sc, especially IL-6, which promotes Th17 differentiation as well as suppresses Th1 differentiation in favor of Th2 development.
Conclusion
Our Sf cytokine measurements reflect a local immune event. In contrast, former study results on T-helper cell populations in both Sf and Sp tend to demonstrate a systemic immune process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Seroma formation after breast surgery is associated with an inflammatory immune response resulting in an increase of Th2/17 associated cytokines. |
Introduction
The formation of a seroma is a very common postoperative complication seen after breast and axillary surgery, especially in case of a mastectomy [1,2,3,4]. To avoid seroma formation, the origin and composition must first be understood, to develop avoidance strategies. The current level of knowledge remains low: seroma formation is defined as a subcutaneous accumulation of fluid, of which the origin is unknown and still controversially discussed. The fluid collection was originally considered as a result of a pure lymphatic obstruction, whereas other studies show characteristics of an exudate [5,6,7,8]. Comparing all of those studies, the time point of fluid analysis seems to be of high importance.
A recent study by our group analyzed the cell content of the seroma fluids and found a specific immunological response through certain T-helper (Th) cell subpopulations. Significantly higher numbers of Th2 and Th17 cells were found in seroma fluid and peripheral blood of the same patients compared to peripheral blood of healthy controls [9]. As known from literature, activated Th cells can differentiate into several functional classes based on their surrounding environment, namely antigen presenting cells (APCs), cytokines, and other costimulatory molecules [10]. Cytokines are known for their immunomodulatory capabilities and are a large and diverse family of small proteins or glycoproteins. Additionally, it was shown that they play a role in developmental processes separate from the immune system, such as cell differentiation and directed migration. Therefore, influencing both, innate and adaptive immune responses. Cytokines are mainly produced by Th cells or macrophages, although they can be transiently induced and secreted by virtually all nucleated cells [11]. In our first publication on seroma immune cell composition, we were able to differentiate Th2/Th17 cells by flow cytometry in seroma fluid (Sf) and peripheral blood, but the measurement of specific cytokines allows a broader picture of T-helper cell differentiation found in Sf.
The Th1/Th2 cell subclass model was discovered based on differential cytokine production and surface marker expression in the 1980s [12]. The Th1/Th2 cell model has many inconsistencies, because human cytokines are rarely exclusive pro-Th1 or -Th2. Therefore, a modern multiplex cytokine measurement approach allows to identify a broad spectrum of cytokines on one hand but also relate these cytokines to Th1 or Th2 cell compositions, as well as to the Th9, Th17, and Th22 cell model, developed decades later [13, 14]. Differentiation into Th2 effector cells is dependent on the presence of Interleukin (IL)-4 [15, 16]. A positive-feedback loop is supported by autocrine IL-4 secretion of Th2 cells, and also they express IL-5 and IL-13 as their signature cytokines [16]. IL-4 combined with IL-6 from APCs suppress Th1 differentiation and modulate the Th1/Th2 balance toward Th2 [15,16,17,18,19]. Protective immune responses orchestrated by Th2 cells aim to target helminths and facilitate tissue repair, but can also contribute to chronic inflammatory diseases, including asthma and allergy [20]. Besides others, Th17 cells are described by the production of IL-10, IL-17, and IL-22 [10]. Polarization proceeds through TGFβ and IL-6, and autocrine amplification by IL-21 production and secretion of IL-23 from APCs stabilizes the Th17 lineage [15, 16, 18].
Based on the lack of scientific knowledge about seroma formation after breast cancer surgery, there is a demand for unraveling the underlying mechanisms of seroma formation for early detection, possible prevention, and/or better treatment strategies. Therefore, one of the aims of the pilot phase of our study was to analyze the cytokine content of collected Sf in patients with simple mastectomy after fine needle aspiration with the help of a multiplex cytokine approach. Simultaneously cytokine levels have been measured in serum of seroma patients (Sp) as well as serum of the healthy controls (Sc). Another aim of this study was to compare certain cytokines measured in this study with the specific Th2/Th17 cell response found in Sf in our former study [9].
Materials and methods
Patients
A first interim analysis of the monocentric pilot study of the international multicenter SerMa study planned to start in July 2023 (EUBREAST 5), where the study concept was validated, included a subgroup population of patients with a surgical procedure of a simple mastectomy with or without sentinel lymph-node excision (SLNE) or/and axillary lymph-node dissection (ALND) and development of a puncturable seroma. SLNE was only performed in patients with invasive disease, cN0 (clinically not affected axillary lymph nodes) and DCIS due to the indication for a mastectomy. All patients presented with invasive breast cancer or DCIS. 34 seroma samples were collected using needle aspiration. In five patients, more than one fine needle aspiration was performed. All the patients gave an informed consent and an ethical committee permitted the study.
Sample processing
Sf and Sp as well as Sc were transported to the laboratory and processed within 24 h. After centrifugation of the Sf, the supernatant was carefully removed from the cell pellet and stored at − 80 ℃. The serum monovettes from patients and controls were centrifuged for 10 min at room temperature, and the serum was carefully removed and stored at − 80 ℃.
Cytokine assays
A “Bio-Plex Pro human Cytokine Screening Panel 48 plex” was used to determine the concentrations of 48 cytokines according to the manufacturer’s instructions (BioRad, Feldkirchen, Germany). Based on our former flow cytometry results, we analyzed and focused on the Th2 cytokines (IL-4, IL-5, and IL-13), Th17 cytokines (IL-6, IL-17, and IL-10), and since it was not included in the Bio-Plex Pro human Cytokine Screening Panel, the Th17 cytokine IL-22. The latter was determined using a DuoSet ELISA kit (R&D Systems) according to the manufacturer’s instructions. Undiluted samples were used and the absorbance was measured at 450 nm with a plate reader (Tecan Spark, Männedorf, Switzerland). Results were evaluated by comparing the measured absorbance after blank reduction to a standard curve provided in the kit.
Statistical analysis
Statistical and correlation analysis was performed using GraphPad Prism. Results were analyzed by either a one-way-ANOVA or Kruskal–Wallis test. A value of p < 0.05 was considered statistically significant.
Results
Patient cohort
A subset of 16 patients treated between 10/2020 and 09/2021 and fulfilling the inclusion criteria (two with bilateral disease) were selected. Simple mastectomy was performed in all of the patients, also in cases with very small invasive tumors and a large concomitant DCIS. SLNE was performed in 12 patients (two both sided) including one DCIS due to the indication to mastectomy. ALND was done in 5 cases (one after SLNE and four due to multiple pathological lymph nodes). Median age was 73 (48–85). Seven of those patients had neoadjuvant chemotherapy. Radiation and endocrine therapy have been done following the general guideline recommendations [21]. The cohort of healthy volunteers consisted of 15 women with a median age of 38 (20–54). Tumor characteristics of the study population are described in Table 1.
Cytokine profile in Sf and serum
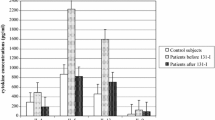
The Th2 differentiation-related cytokine IL-4 was detected in significantly higher amounts in Sf (12.84 ± 4.59 pg/ml) compared to Sp (3.3 ± 0.83 pg/ml) and Sc (2.65 ± 0.7 pg/ml). In addition to IL-4, mature Th2 cells secrete IL-5 and IL-13. We found significantly higher amounts of IL-5 in Sf (131.5 ± 96.6 pg/ml) compared to serum were IL-5 was nearly absent (Sp: 4.6 ± 12.2 pg/ml; Sc: 0.0 pg/ml). IL-13 levels show the same pattern, although total amounts in Sf (2.2 ± 1.5 pg/ml; Sp: 0.2 ± 0.6 pg/ml; Sc: 0.1 ± 0.3 pg/ml) were lower compared to IL-5 (Fig. 1A).
Cytokine level in pg/ml measured with the Bio-Plex platform or ELISA. A Cytokine important for the differentiation of Th2 cells, IL-4, and Th2 cell-secreted cytokines IL-5 and IL-13 in pg/ml. B Cytokine important for the differentiation of Th17 cells, IL-6, and cytokines secreted by Th17 cells: IL-17, IL-10, and IL-22 in pg/ml. *p < 0.05, ***p < 0.001, ****p < 0.0001
One important cytokine for the differentiation of naïve Th cells into Th17 cells is IL-6. We measured up to 10.000 fold higher levels of IL-6 in Sf (6.2 ± 7.9 ng/ml), which is highly significant compared to Sp (1.9 ± 4.1 pg/ml) and Sc (0.1 ± 0 pg/ml), where IL-6 is not detectable in most samples (Fig. 1B). Cytokines secreted by Th17 cells, IL-17, and IL-10 have also been found in significantly higher levels in Sf than in serum (Sp and Sc). In contrast, IL-22, also a secretion product of Th17 cells, was found in significantly lower levels in Sf (234.6 ± 365.9 pg/ml) compared to Sp (318.7 ± 295.9 pg/ml). Compared to Sc (252.8 ± 319.7 pg/ml), we could not see a significant difference.
Comparative analyses of cytokine measurements and cellular content of Th2/Th17 cells
Within this study, we focused on Th2 and Th17 cytokines. Based on the results of our former flow cytometry analyses of the cellular Sf contend, we were able to compare both measurements. We found a highly significant correlation between the levels of IL-6 as well as IL-10 and the number of Th17 cells (Fig. 2). All other correlations were not significant.
Correlation analysis between cytokines and number of Th2/Th17 cells in Sf. A Correlation between number of Th2 cells [%] and IL-4, IL-5, and IL-13 levels [pg/ml]. No significant correlation was found. B Correlation analysis between Th17 cells [%] and IL-6, IL-17, IL-17, and IL-22 levels [pg/ml]. Significant correlations were found for IL-6 (***) and IL-10 (***). ***p < 0.001
Discussion
These are the first published results of the pilot study of the SerMa study (EUBREAST 5), describing the cytokine content in relation to the Th-cellular content of seroma fluids in our former publication [9]. We elucidated connections between the specific Th2/Th17 immune response by analyzing the cytokine abundance of the collected fluids in comparison to the cellular content. In our former study, we found significantly higher percentages of Th2 cells in seroma fluid and peripheral blood of the patients compared to blood of healthy controls [9]. These results are now supported by the findings of the associated ILs, especially the significantly higher levels of IL-4, the most important cytokine regarding Th2 differentiation, measured in Sf. It is known that B cells, NKT cells, naïve Th cells, and mast cells can secrete IL-4 and, therefore, promote Th2 differentiation [22]. Interestingly, we found significantly lower numbers of B cells, NKT, and naïve Th cells in Sf compared to peripheral blood of patients and healthy controls, excluding those as the origin of IL-4 found in Sf [9]. Therefore, mast cells could potentially be a source of IL-4 in Sf. Additionally, Th2 cells support a positive-feedback loop by an autocrine secretion of IL-4 [16]. Significantly higher Th2 cell percentages in Sf and blood of patients could also be due to the very high levels of IL-6 measured in Sf. We also measured this cytokine in Sf. IL-4 in combination with IL-6 suppresses Th1 differentiation in favor toward Th2 development [15,16,17,18,19]. These increased IL-4 and IL-6 levels support our finding of a strong Th2 cell response in Sf.
In addition, IL-6 is an essential differentiation factor for Th17 cells [23, 24]. There were significantly more Th17 cells in Sf and blood compared to healthy controls [9]. In addition, we performed a correlation analysis between flow cytometry results and Bio-Plex cytokine measurements in Sf. IL-6 is highly abundant in Sf, and we could show a significant positive correlation between Th17 cell percentage and levels of IL-6 in the collected fluids, suggesting a direct connection between IL-6 levels and Th17 cell abundance. For the cytokines secreted by Th17 cells, we measured significantly higher IL-17 amounts in Sf. Interestingly, we found a positive correlation between Th17 cells and the abundance of IL-10, suggesting that most of the Th17 cells found in the Sf secrete IL-10 into their surroundings. It has been shown that different cytokines promoting Th17 differentiation can induce unique types of Th17 cells. TGF-β induces an IL-10-producing and less pathogenic subtype, while IL-1β promotes a more pro-inflammatory type of Th17 cells [16, 25,26,27]. These results verify the previous flow cytometry results by simple Bio-Plex cytokine measurements.
IL-4 serum levels in healthy individuals and in patients did not differ, indicating this cytokine as a rather local immune marker in contrast to Th2 cell, which showed a systemic abundance [9]. There is a study, which showed a global decrease in cytokine serum levels after neoadjuvant chemotherapy [28]. This effect we could not see in serum of our participants who received a neoadjuvant chemotherapy compared to healthy controls. Even though the median age of the control group is lower than our participants. There is evidence that serum cytokine levels could change with age, but for IL-6 and Ll-17, no differences could be found regarding age [29]. IL-6 levels are very high in Sf, but not detectable in Sp and cannot be connected to the higher numbers of Th17 cells found in peripheral blood of patients compared to healthy controls. Additionally, there was no direct correlation between IL-17 levels and Th17 cells numbers in Sf. All these results indicate a locally restricted immune response regarding cytokines rather than a systemic change as seen with the Th2/Th17 cells. These findings must be interpreted with caution in light of the limited number of patient cases and therefore have to be verified in a larger collective.
In summary, we could show that Sf is highly cytokine rich. Whereas, flow cytometry analysis of Th cell subpopulations showed a significantly higher cell abundance in seroma formations as well as peripheral blood of patients compared to healthy controls and, therefore, are rather connected to systemic processes. We found significant cytokine concentration changes in Sf but not in Sp or Sc. Therefore, cytokines were found to be rather locally constricted at the site of seroma formation and not systemically in serum. Further investigations will lead to more precise statements within the prospectively planned international multicenter SerMa study (EUBREAST5).
Conclusions
This study demonstrated a successful determination of different cytokines in postoperatively developed seromas fluids of patients diagnosed with breast cancer and are locally treated with a simple mastectomy with or without an axillary surgical procedure. The Th2 differentiation-related cytokine IL-4 was found in significantly higher concentrations in Sf compared to serum (Sp and Sc). Additionally, we measured very high levels of IL-6 in Sf, also highly significant compared to Sp and Sc. IL-6 is known to promote Th17 differentiation as well as suppressing Th1 differentiation in favor toward Th2 development. Therefore, we were able to explain a concordance of cytokine expression and Th cell differentiation in seroma patients. High cytokine levels in Sf and low in Sp and Sc support a local limited immunological event in seromas.
Data availability statement
Additional data are available from the authors upon request based on ethical requirements.
References
Al-Gaithy ZK, Ayuob NN (2012) Vascular and cellular events in post-mastectomy seroma: an immunohistochemical study. Cell Immunol 272(2):130–136
Andeweg CS, Schriek MJ, Heisterkamp J, Roukema JA (2011) Seroma formation in two cohorts after axillary lymph node dissection in breast cancer surgery: does timing of drain removal matter? Breast J 17(4):359–364
Kumar S, Lal B, Misra MC (1995) Post-mastectomy seroma: a new look into the aetiology of an old problem. J R Coll Surg Edinb 40(5):292–294
Yu J, Olsen MA, Margenthaler JA (2020) Indications for readmission following mastectomy for breast cancer: an assessment of patient and operative factors. Breast J 26(10):1966–1972
Tejler G, Aspegren K (1985) Complications and hospital stay after surgery for breast cancer: a prospective study of 385 patients. Br J Surg 72(7):542–544
Watt-Boolsen S, Nielsen VB, Jensen J, Bak S (1989) Postmastectomy seroma. A study of the nature and origin of seroma after mastectomy. Dan Med Bull 36(5):487–489
O’Dwyer PJ, O’Higgins NJ, James AG (1991) Effect of closing dead space on incidence of seroma after mastectomy. Surg Gynecol Obstet 172(1):55–56
Bonnema J, Ligtenstein DA, Wiggers T, van Geel AN (1999) The composition of serous fluid after axillary dissection. Eur J Surg 165(1):9–13
Pochert N, Schneider M, Ansorge N, Strieder A, Sagasser J, Reiger M, Traidl-Hoffmann C, Neumann A, Jeschke U, Dannecker C, Kuhn T, Ditsch N (2022) Seroma after simple mastectomy in breast cancer-the role of CD4+ T helper cells and the evidence as a possible specific immune process. Int J Mol Sci 23(9):4848
Basu A, Ramamoorthi G, Albert G, Gallen C, Beyer A, Snyder C, Koski G, Disis ML, Czerniecki BJ, Kodumudi K (2021) Differentiation and regulation of TH cells: a balancing act for cancer immunotherapy. Front Immunol 12:669474
Commins SP, Borish L, Steinke JW (2010) Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol 125(2 Suppl 2):S53-72
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136(7):2348–2357
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421(6924):744–748
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13(5):715–725
Luckheeram RV, Zhou R, Verma AD, Xia B (2012) CD4(+)T cells: differentiation and functions. Clin Dev Immunol 2012:925135
Saravia J, Chapman NM, Chi H (2019) Helper T cell differentiation. Cell Mol Immunol 16(7):634–643
Dobrzanski MJ (2013) Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy. Front Oncol 3:63
Zhu J (2018) T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol 10(10):a030338
Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA (1997) Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med 185(3):461–469
Walker JA, McKenzie ANJ (2018) TH2 cell development and function. Nat Rev Immunol 18(2):121–133
Ditsch N, Wocke A, Untch M, Jackisch C, Albert US, Banys-Paluchowski M, Bauerfeind I, Blohmer JU, Budach W, Dall P, Fallenberg EM, Fasching PA, Fehm TN, Friedrich M, Gerber B, Gluz O, Harbeck N, Heil J, Huober J, Kreipe HH, Krug D, Kuhn T, Kummel S, Kolberg-Liedtke C, Loibl S, Luftner D, Lux MP, Maass N, Mundhenke C, Nitz U, Park-Simon TW, Reimer T, Rhiem K, Rody A, Schmidt M, Schneeweiss A, Schutz F, Sinn HP, Solbach C, Solomayer EF, Stickeler E, Thomssen C, Witzel I, Muller V, Janni W, Thill M (2022) AGO recommendations for the diagnosis and treatment of patients with early breast cancer: update 2022. Breast Care (Basel) 17(4):403–420
Kumar S, Jeong Y, Ashraf MU, Bae YS (2019) Dendritic cell-mediated Th2 immunity and immune disorders. Int J Mol Sci 20(9):2159
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT (2006) Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441(7090):231–234
Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F (2012) Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 484(7395):514–518
Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ (2010) Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467(7318):967–971
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8(12):1390–1397
Jabeen S, Zucknick M, Nome M, Dannenfelser R, Fleischer T, Kumar S, Luders T, von der Lippe Gythfeldt H, Troyanskaya O, Kyte JA, Borresen-Dale AL, Naume B, Tekpli X, Engebraaten O, Kristensen V (2018) Serum cytokine levels in breast cancer patients during neoadjuvant treatment with bevacizumab. Oncoimmunology 7(11):e1457598
Kim HO, Kim HS, Youn JC, Shin EC, Park S (2011) Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med 9:113
Acknowledgements
The authors would like to thank C. Kuhn for technical support.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by a research grant of the Medical Faculty of the University Augsburg (“Projektförderung—intramurale Forschungsförderung Medizinische Fakultät”) for N.D.
Author information
Authors and Affiliations
Contributions
Conceptualization: UJ and ND; methodology: CT-H; software: CT-H; validation: MS, MK, and AM; formal analyses: CD; investigation: MMG and NP; resources: MBP and ND; data curation: TK and MU; writing—original draft preparation: NP; writing—review and editing: ND; visualization: TK; supervision: UJ; project administration: ND; funding acquisition: ND. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
N.D. reports funding from MSD, Novartis, Pfizer, Roche, AstraZeneca, TEVA, Mentor, and MCI Healthcare. C.D. is funded by Roche, AstraZeneca, TEVA, Mentor, and MCI Healthcare. J.S. is funded by Novartis, Roche, Pfizer, BMS, and AstraZeneca. M.U. reports personal fees and non-financial support from Abbvie, personal fees and non-financial support from Amgen GmbH, personal fees and non-financial support from AstraZeneca, personal fees from BMS, personal fees and non-financial support from Celgene GmbH, personal fees and non-financial support from Daiji Sankyo, personal fees and non-financial support from Eisai GmbH, personal fees from Lilly Deutschland, personal fees and non-financial support from Lilly Int., personal fees and non-financial support from MSD Merck, personal fees and non-financial support from Mundipharma, personal fees and non-financial support from Myriad Genetics, personal fees and non-financial support from Odonate, personal fees and non-financial support from Pfizer GmbH, personal fees from PUMA Biotechnology, personal fees and non-financial support from Roche Pharma AG, personal fees and non-financial support from Sanofi Aventis Deutschland GmbH, personal fees and non-financial support from TEVA Pharmaceuticals Ind Ltd., personal fees and non-financial support from Novartis, personal fees from Pierre Fabre, personal fees and non-financial support from Clovis Oncology, and personal fees from Seattle Genetics, outside the submitted work. M.B.P. got funding from Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, Canon, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Pintuition, Pierre Fabre, and ExactSciences. T.K. was funded by Endomag, Hologic, Mammotome, Merit Medical, Pfizer, Sirius Medical, Pfizer, Astra Zeneca, MSD, Gilead, Exact Sciences, and Daiichi-Sankyo. All other authors report no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pochert, N., Schneider, M., Köpke, M.B. et al. Th2/Th17 cell associated cytokines found in seroma fluids after breast cancer surgery. Arch Gynecol Obstet 308, 1621–1627 (2023). https://doi.org/10.1007/s00404-023-07074-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07074-w