Abstract
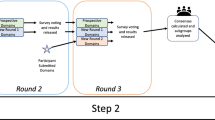
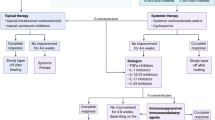
Pyoderma gangrenosum (PG) is a rare inflammatory condition with an immense disease burden that remains understudied. With limited approved treatments and low-quality clinical evidence, PG continues to have poor patient outcomes. Unfortunately, improvement in PG treatments and patient care is based on additional research endeavors that can only be developed from existing high-quality data. The following protocol outlines the development of the Minimum Data Set for Treatment Effectiveness in Pyoderma gangrenosum (MIDSTEP), a core set of domains and domain items for the Pyoderma Gangrenosum Treatment Effectiveness (PyGaTE) international registry. The outcomes and benefits are focused on providing real-world data for physicians to improve their clinical decisions on PG treatment and inform clinical trial design, promoting clinical research among the international scientific community. MIDSTEP is a multi-phase project. The first phase will produce a domain item list from a literature review to take into the second phase which would finalize the core data set by an e-Delphi exercise. There will be a single stakeholder group participating together in the e-Delphi consisting of PG experts (healthcare providers, researchers, methodologists, industry representatives, and regulators), ulcerative PG patients, and PG patient advocates. The methodology outlined in the protocol is a systematic method based on several guidelines through COMET and established dermatologic registries and outcome sets with systematic methodologies of their own. The third phase will identify the instruments for the items, the ‘when to measure’ the items, and the platform for the registry. The last phase is the implementation and continued maintenance of the international registry PyGaTE. By solidifying a consensus on standardized outcomes and collecting information on PG treatment effectiveness in a centralized database, existing treatments can be compared more systematically and analyzed with increased evidence. MIDSTEP and the PyGaTE international registry will have the ambitious goal to generate and disseminate real-world data that can be used by all stakeholders to improve health outcomes for PG patients. Future potential for the outcome of this project includes the development of a gold-standard PG treatment.

Similar content being viewed by others
Data availability
No data was used for the research described in the article.
References
Barrett D, Heale R (2020) What are Delphi studies? Evid Based Nurs 23(3):68–69. https://doi.org/10.1136/ebnurs-2020-103303
Brooklyn TN, Dunnill MG, Shetty A et al (2006) Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut 55(4):505–509. https://doi.org/10.1136/gut.2005.074815
Chalmers JR, Schmitt J, Apfelbacher C et al (2014) Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 171(6):1318–1325. https://doi.org/10.1111/bjd.13237
Choquet R, Maaroufi M, de Carrara A, Messiaen C, Luigi E, Landais P (2015) A methodology for a minimum data set for rare diseases to support national centers of excellence for healthcare and research. J Am Med Inform Assoc 22(1):76–85. https://doi.org/10.1136/amiajnl-2014-002794
De Meyer D, Kottner J, Beele H et al (2019) Delphi procedure in core outcome set development: rating scale and consensus criteria determined outcome selection. J Clin Epidemiol 111:23–31. https://doi.org/10.1016/j.jclinepi.2019.03.011
Gameiro A, Pereira N, Cardoso JC, Gonçalo M (2015) Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 8:285–293. https://doi.org/10.2147/CCID.S61202. (Published 2015 May 28)
George C, Deroide F, Rustin M (2019) Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond) 19(3):224–228. https://doi.org/10.7861/clinmedicine.19-3-224
Gerbens LAA, Boyce AE, Wall D et al (2017) TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema registries. Trials 18:87. https://doi.org/10.1186/s13063-016-1765-7
Giannuzzi V, Stoyanova-Beninska V, Hivert V (2022) Editorial: The use of real world data for regulatory purposes in the rare diseases setting. Front Pharmacol 13:1089033. https://doi.org/10.3389/fphar.2022.1089033. (Published 2022 Nov 24)
Gliklich RE, Dreyer NA, Leavy MB (eds) (2014) Registries for evaluating patient outcomes: a user’s guide, 3rd edn. Agency for Healthcare Research and Quality, Rockville
Homs-Romero E, Romero-Collado A (2019) Development of a minimum data set registry for chronic venous insufficiency of the lower limbs. J Clin Med 8(11):1779. https://doi.org/10.3390/jcm8111779. (Published 2019 Oct 24)
Ighani A, Al-Mutairi D, Rahmani A, Weizman AV, Piguet V, Alavi A (2019) Pyoderma gangrenosum and its impact on quality of life: a multicentre, prospective study. Br J Dermatol 180(3):672–673. https://doi.org/10.1111/bjd.17347
Jockenhöfer F, Wollina U, Salva KA, Benson S, Dissemond J (2019) The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol 180(3):615–620. https://doi.org/10.1111/bjd.16401
Kempf L, Goldsmith JC, Temple R (2018) Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A 176(4):773–783. https://doi.org/10.1002/ajmg.a.38413
Kottner J, Jacobi L, Hahnel E et al (2018) Core outcome sets in dermatology: report from the second meeting of the International Cochrane Skin Group Core Outcome Set Initiative. Br J Dermatol 178(4):e279–e285. https://doi.org/10.1111/bjd.16324
Lange T, Kopkow C, Lützner J et al (2020) Comparison of different rating scales for the use in Delphi studies: different scales lead to different consensus and show different test-retest reliability. BMC Med Res Methodol 20(1):28. https://doi.org/10.1186/s12874-020-0912-8. (Published 2020 Feb 10)
Lee S, Lee JY, Ju HJ et al (2023) Association of all-cause and cause-specific mortality risks with pyoderma gangrenosum. JAMA Dermatol 159(2):151–159. https://doi.org/10.1001/jamadermatol.2022.5437
Maronese CA, Pimentel MA, Li MM, Genovese G, Ortega-Loayza AG, Marzano AV (2022) Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol 23(5):615–634. https://doi.org/10.1007/s40257-022-00699-8
Maverakis E, Marzano AV, Le ST et al (2020) Pyoderma gangrenosum. Nat Rev Dis Primers 6(1):81. https://doi.org/10.1038/s41572-020-0213-x. (Published 2020 Oct 8)
McKenzie F, Cash D, Gupta A, Cummings LW, Ortega-Loayza AG (2019) Biologic and small-molecule medications in the management of pyoderma gangrenosum. J Dermatol Treat 30(3):264–276. https://doi.org/10.1080/09546634.2018.1506083
NCATS Division of Rare Diseases Research Innovation (2022) Rare diseases registry program (radar). National Center for Advancing Translational Sciences. https://ncats.nih.gov/radar#:~:text=A%20registry%20is%20a%20collection,and%20support%20patient%2Dfocused%20research. Accessed 11 May 2023
Orfaly VE, Reese AM, Friedman M, Latour E, Ortega-Loayza AG (2022) Pyoderma gangrenosum study pilot registry: the first step to a better understanding. Wound Repair Regen 30(3):334–337. https://doi.org/10.1111/wrr.13005
Pizzamiglio C, Vernon HJ, Hanna MG, Pitceathly RDS (2022) Designing clinical trials for rare diseases: unique challenges and opportunities. Nat Rev Methods Primers. https://doi.org/10.1038/s43586-022-00100-2. (Published 2022 Mar 10)
Rick J, Gould LJ, Marzano AV et al (2023) The “understanding pyoderma gangrenosum, review and assessment of disease effects (UPGRADE)” Project: a protocol for the development of the core outcome domain set for trials in pyoderma gangrenosum. Arch Dermatol Res 315:983–988. https://doi.org/10.1007/s00403-022-02424-1
Tan MH, Thomas M, MacEachern MP (2015) Using registries to recruit subjects for clinical trials. Contemp Clin Trials 41:31–38. https://doi.org/10.1016/j.cct.2014.12.012
TREAT Registry Taskforce (2018) Projects. TREAT Registry Taskforce. https://treat-registry-taskforce.org/projects/#. Accessed 25 June 2023
Wall D, Meah N, York K et al (2021) A global eDelphi exercise to identify core domains and domain items for the development of a global registry of alopecia areata disease severity and treatment safety (GRASS). JAMA Dermatol 157(4):1–11. https://doi.org/10.1001/jamadermatol.2020.5839
Williamson PR, Altman DG, Bagley H et al (2017) The COMET handbook: version 1.0. Trials 18(Suppl 3):280. https://doi.org/10.1186/s13063-017-1978-4. (Published 2017 Jun 20)
Author information
Authors and Affiliations
Contributions
Author OMH wrote the original draft, prepared figure 1, led investigation and participated in project administration and methodology. Author MEJ participated in review & editing and investigation and contributed to methodology. Authors AGOL and LAAG participated in review and editing and were responsible for the project conceptualization and contributed to methodology. DC, DOC, JD, JRVG, PJH, RIK AVM, and YT participated in review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Diana Chen is an employee of Genentech, a member of the Roche Group. Dr. David Croitoru worked as a consultant for UCB, Bausche, Novartis, Abbvie and Sanofi. Dr. Angelo Marzano receives consultation and advisory boards disease-relevant honoraria from AbbVie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi, and UCB. Dr. Yayoi Tada receives research grants and honoraria from Eisai, AbbVie, and Novartis. Dr. Alex Ortega-Loayza is president-elect of Pacific Dermatologic Association, an associate editor of Dermatology (Karger), a consultant for Genentech, Guidepoint, Corvus Pharmaceuticals, and Castle Biosciences, on the advisory board of Bristol Meyer Squibb, Boehringer Ingelheim, and Janssen, and receives research grants from AbbVie, Lilly, Janssen, Pfizer, and InflaRx GmbH. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1. Literature search strategy
Appendix 1. Literature search strategy
Database: Ovid MEDLINE(R) ALL < 1946 to May 23, 2023 >
-
1.
exp Pyoderma Gangrenosum/ (2571)
-
2.
(pyoderm* adj3 gangren*).ab,hw,kf,ot,sy,ti,fx,nm,ox,px,rx,ui. (4098)
-
3.
1 or 2 (4098)
-
4.
limit 3 to (meta analysis or "systematic review") (53)
-
5.
limit 3 to (controlled clinical trial or randomized controlled trial) (9)
-
6.
exp epidemiologic studies/ (3125284)
-
7.
3 and 6 (355)
-
8.
4 or 5 or 7 (414)
-
9.
limit 8 to english language (382)
-
10.
limit 8 to abstracts (346)
-
11.
9 or 10 (413)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haddadin, O.M., Jacobson, M.E., Chen, D.M. et al. Minimum data set for treatment effectiveness in pyoderma gangrenosum (MIDSTEP): an international protocol of an e-Delphi study to develop a clinical physician-driven treatment effectiveness registry on behalf of the UPGRADE initiative. Arch Dermatol Res 315, 2913–2919 (2023). https://doi.org/10.1007/s00403-023-02729-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-023-02729-9