Abstract
Acanthosis nigricans is characterized by the presence of velvety hyperpigmentation of the skin over the neck and the flexural areas. Regardless of different modalities of treatment, none provides a definite standard cure. This study aims to assess the efficacy of topical 10% salicylic acid compared to 10% urea cream in treating acanthosis nigricans in adolescents. A randomized comparative, double-blind study is conducted on adolescents with acanthosis nigricans of the posterior neck. Treatment efficacy was assessed via a narrowband reflectance spectrophotometer at individual follow-up visits at weeks 2, 4, and 8, while the overall success rates were evaluated by the investigator-assessed and participant-assessed global evaluation scales (IGE and PGE). Acanthosis nigricans scoring chart (ANSC) and adverse effects are also assessed. A total of 39 participants with acanthosis nigricans enrolled and completed the study. Throughout the 8-week period of treatment, the use of 10% salicylic acid demonstrated strong effectiveness in treatment with 14.6 ± 10.6% improvement, while the 10% urea demonstrated 12.5 ± 10.9% improvement. Findings from the overall global evaluation scales were consistent with the results from the narrowband reflectance spectrophotometer. Treatment with 10% salicylic and 10% urea produced no serious local skin adverse reactions. Both medications improve neck hyperpigmentation associated with acanthosis nigricans in adolescents, in which the 10% salicylic acid and 10% urea cream demonstrate similar efficacy and safety profiles. Clinical Trials Registry: TCTR20201123003.
Similar content being viewed by others
Introduction
Acanthosis nigricans (AN) is a skin condition characterized by the darkening and thickening of the skin found mostly in the flexural areas, particularly in the neck, axilla, and groins [1]. AN can be commonly found in both pediatric and adult patients; the skin disorder is closely associated with obesity, type II diabetes mellitus, and insulin resistance. The pathogenesis of AN is most related to the activation of insulin and insulin-like growth factor (IGF) on keratinocytes, which leads to increased stimulation of epidermal keratinocytes [2]. With rising diabetes, obesity, and other non-communicable diseases among the world population, AN prevalence is currently increasing which varies according to the degree of obesity, age, race, and other endocrine issues [2]. The main concerns for patients are often cosmetic-related but the hyperpigmentation may cause distress as they could be signs of other underlying diseases; therefore, effective management is essential to reduce skin hyperpigmentation.
Although various therapeutic approaches to AN are available, a single unit of universal standard treatment is not yet established. The approach is to treat the underlying disease, weight management in obesity, avoidance of drug-induced AN, application of topical agents, and cosmetic surgery [1]. The potential of topical agents in the treatment with AN involves interfering with keratinocyte proliferation. Urea cream, a carbamide-containing cream, has been studied to be a potent emollient and possesses a proteolytic effect and keratolysis, leading it to be an effective therapy for conditions associated with dry skin [3, 4]. Although urea may be inferior to 0.025% tretinoin, it significantly improves skin hyperpigmentation, thus showing promising results as different preparation of urea creams have useful properties, such as keratolysis and even tissue softeners for nails and/or skin [3]. Another agent which is used to treat many skin disorders is salicylic acid. With the ability to exfoliate the stratum corneum, salicylic acid is considered a keratolytic agent that is used in many conditions, such as melasma, photodamage, and freckles [5]. The mechanism of chemical peeling involves injury to the skin to rejuvenate, leading to improvement of skin texture and smoothening of skin. Another topical cream that has a similar mechanism of action is tretinoin and adapalene, which can cause an improvement of AN lesions at the posterior neck [3, 6]. Currently, there are limited clinical trials available to determine the efficacy and safety of topical salicylic acid. Therefore, the aim of this study is to assess the efficacy of 10% salicylic acid cream compared to 10% urea cream with the treatment of AN.
Subjects and methods
Study design
This study spans a total of eight weeks as a randomized, double-blinded, comparative approach to compare the efficacy and safety of 10% salicylic acid cream and 10% urea creams for treating AN. Recruited participants were studied at HRH Princess Maha Chakri Sirindhorn Medical Center in Thailand. The participants were randomly allocated to two groups by simple randomization using a third investigator, who was not directly involved in data analysis. Instructions for the participants were given with the jars containing the topical creams either 10% salicylic acid or 10% urea without official labels to prevent bias. On the first visit, participants underwent a thorough physical examination, including body weight (kg), height (cm), body mass index (BMI), skin color, and the Fitzpatrick skin phototypes. The study used the criteria for Body Mass Index (BMI) for age from the Centers for Disease Control and Prevention (CDC), in which participants were categorized into healthy weight (5th to < 85th percentile), overweight (85th to < 95th percentile) and obesity (95th percentile or greater). One gram of cream was applied to each side of the posterior neck twice daily. The patient’s compliance was assessed by weighing the jars containing the cream at every visit and the weight was recorded. To fully assess the effects of the topical creams, other agents were not prescribed, and the participants were advised not to use other topicals on the posterior neck or be exposed to long periods of sunlight. If cutaneous irritations were to occur, the participants could stop for three days before continuing the cream usage. Temporary treatment discontinuation by the participants was also recorded, along with the adverse effects experienced. Participants were permitted to contact the investigators if severe side effects unfortunately occurred. Intermittent follow-up visits were scheduled for week 2, 4, and 8 with the same blinded investigator. The study has been approved by the Institutional Review Board and Ethics Committee of Srinakharinwirot University. Its protocol has been registered with the Thai Clinical Trials Registry (TCTR20201123003).
Subjects
The inclusion criteria for participants in the study were adolescents aged 12–18 years who were clinically diagnosed with AN at the posterior neck. The exclusion criteria included subjects with vulnerable skin diseases, infection, skin lesions, tattoos, serious comorbidities (type II diabetes mellitus, hypertension, liver disease, kidney disease, coagulopathy), current use of oral retinoids, history of photoallergy, immunocompromised hosts, current breastfeeding, and history of applying any topical agents such as corticosteroid, vitamin D, or trichloroacetic acid (TCA) within four weeks to the studied area.
The estimated sample size was calculated from an expected effect size of 0.3, an alpha level of 0.05, and a power of 0.8. The participants were separated into two groups with 20 people each, assuming a 10% dropout rate. Additionally, participants were given the choice of withdrawing from the study anytime in the event of any allergic reaction, erythema, and other unwanted side effects that may affect the patient’s quality of life.
Outcome assessment
The primary outcome was the efficacy in reducing skin pigmentation by using Mexameter MX 18 (Courage and Khazaka Electronic, Cologne, Germany), which is narrowband reflectance spectrophotometry, to evaluate changes in melanin (M) and erythema (E) indices in week 0, 2, 4 and 8. As the values of M (darkness) and E (redness) indices, the numbers correlate with the darkness and redness of the skin. At each visit, three readings were taken consecutively from each side of the participant’s neck. To ensure the same location was assessed at every follow-up visit, a personalized transparent sheet sized 10 × 20 cm, with 2 × 2 cm rectangular holes on each side of the neck midline was utilized to measure skin pigmentation with the lower border as the C7 spinous level. Moreover, skin pigmentation was measured as a reference at the participant’s back by using the same protocol with draping holes on a transparent sheet. A single trained investigator was involved in spectrophotometric measurement to ensure controlled probe pressure under fluorescent lighting.
The secondary outcomes were from assessing the overall investigator and patient global evaluation scales (IGE and PGE), improvement in scores of acanthosis nigricans scoring chart (ANSC) (Fig. 1), and adverse cutaneous reactions from treatment using cutaneous irritation grading scales. IGE and PGE scales were evaluated by a trained investigator, with scores ranging from 0 to 6 (0 = clear, 1 = almost clear or > 90% improvement; 2 = marked improvement or 75% improvement; 3 = moderate improvement or 50% improvement; 4 = mild improvement or 25% improvement; 5 = no change; 6 = worsening). The patient’s adverse reactions were also recorded through the use of the cutaneous irritation grading scales, as participants evaluated erythema, dryness, peeling, burning, and itching on a scale of one to four (one representing no adverse reaction, four as marked severity). As mentioned earlier, ANSC is an in-house assessment tool measuring the progression of AN after treatment. By having specific descriptions and images of the skin in the ANSC, this tool ensures objectivity among the physicians to accurately rate the skin color and texture – scores are allocated for each parameter of skin color and texture from one to eight and one to six respectively to calculate the total ANSC score. Calculating the scores for texture demonstrates excellent intra-and interrater reliabilities (ICC > 0.85) and correlates with narrowband reflectance spectrophotometry (r > 0.6).
Statistical analysis
The analyses were conducted using SPSS version 26 (IBM, New York). Categorical data, such as sex, skin phototypes criteria, cutaneous irritation, global evaluation score, and cutaneous irritation grading scales were described as percentages. Continuous data, such as age, height, and BMI, were presented as mean (standard deviation). As every individual contributed two data sets (left and right side of the posterior neck), the measurements from each side of the neck were only considered as one data set. The treatment efficacy was assessed by comparing the differences prior to and after receiving the treatment using paired t-test (p < 0.05). The Kruskal–Wallis test compared the IGE, PGE, and adverse cutaneous irritation grading scales. The comparison of treatment in different time points from week 0, 2, 4, and 8 for both 10% urea cream and 10% salicylic acid was performed using repeated measure analysis of variance (ANOVA).
Results
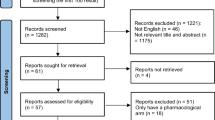
A total of 40 participants were recruited, and 39 completed the study. Unfortunately, one participant was infected with Covid-19 in the first week of enrolment and thus requested to be withdrawn from the study. In each group of 10% salicylic acid and 10% urea, the mean (SD) age was 13.74 (1.79) and 14.95 (1.67), respectively. In terms of the baseline characteristics of participants, 73.6% of the 10% salicylic group and 50.0% of the 10% urea group were males. In determining Fitzpatrick skin phototype, there were 63.2% of the 10% salicylic acid group and 50.0% of the 10% urea group with type V. Of 89.5% and 75% of participants were accordingly diagnosed with obesity in each respective group (Table 1).
Primary outcome
During the study duration, both treatments demonstrated efficacy in improving neck hyperpigmentation by the M index measurement (p < 0.001). The mean reduction of the M index between week 0 and week 8 of the treatment with 10% salicylic acid was 119.3 (95% CI 90.9, 147.7), which is proportional to 14.6 ± 10.6% improvement, whereas the 10% urea cream revealed a 93.6 reduction (95% CI 68.1, 119.1) with 12.5 ± 10.9% improvement (Table 2 and Fig. 2). Additional analysis using repeated measures ANOVA revealed that the result has no significant differences in treatment efficacy between groups on each consecutive follow-up. Likewise, the E index of the neck and the M and E indices of the back showed no significant changes at the end of the study (Table S1). Clinical pictures of participants in both groups of treatment are shown in Fig. 3.
Secondary outcome
Improvement of neck hyperpigmentation assessed by ANSC showed significant improvement from week 0 to 8 in both groups (p < 0.001), which was consistent with the results from spectrophotometry. From week 0 to week 8, changes in the total scores in the 10% salicylic and the 10% urea groups were 23.4 ± 8.9% and 19.9 ± 13.7% respectively. The secondary outcomes were assessed by IGE, and slight improvement was reported in both groups during the early treatment period. Nevertheless, the 10% salicylic revealed significantly better efficacy in neck hyperpigmentation improvement than 10% urea by week 8 (Table S2). For PGE assessment, 89.4% of patients in 10% salicylic groups rated more than 75% improvement of their skin pigmentation, whereas 60.0% of patients in 10% urea groups rated more than 75% improvement.
Local cutaneous adverse effects
Both treatments were well tolerated by participants during the follow-up period with no severe reactions. In terms of adverse events, a few participants in the 10% salicylic experienced slightly to mild dryness (10.5%), peeling (26.3%), and burning (5.3%) in the first 2 weeks. In week 4, 5.3% of participants still experienced peeling. While the 10% urea cream showed no cutaneous irritation.
Discussion
This double-blind, randomized, and controlled trial established profound benefits in both treatment options, in which both 10% salicylic and 10% urea creams significantly improved the hyperpigmented lesion of the neck from week 0 to 8. Although the 10% salicylic revealed a better efficacy in neck hyperpigmentation improvement than the 10% urea cream, the treatment outcomes between the groups were not statistically different. On the account of no standard tools for AN assessment, our study incorporated both objective and subjective parameters for assessments to ensure the reliability of trial outcomes. The reading from spectrophotometry, global evaluation score, and ANSC demonstrated the congruence of outcome which 10% salicylic acid had a better clearance of lesion. The results of 10% urea cream on neck hyperpigmentation improvement were also consistent with previous reports [3, 7]. Although the skin peeling effect of the salicylic acid was beneficial for neck hyperpigmentation improvement, retinoid therapy such as tretinoin and adapalene showed higher efficacy than 10% salicylic acid, when comparing data with previous studies [3, 6].
With regards to the tolerability of the treatments, topical 10% salicylic creams may cause mild irritation of the skin, which is characterized by dry skin, scaling, and burning. These symptoms occurred only for a few weeks early in the study and patients gradually lose these symptoms over time as the trial continued. The results of adverse events were comparable to retinoid treatment which is associated with the exfoliation of the skin. In terms of local adverse reactions, 10% urea creams have generally very mild side effects, such as mild drying and peeling [3, 7], but participants in the study reported none of such reactions. Besides, topical agents and several treatment options include oral retinoids, which may be an effective remedy for AN [8]. Oral antihyperglycemic agents such as rosiglitazone and metformin also revealed satisfactory improvement of AN skin lesions associated with insulin resistance [9]. However, the goal of therapy and treatment direction in obesity-associated AN should always focus on weight reduction which may resolve hyperkeratotic lesions. Other potential treatments such as erbium fiber laser have been reported to be used in patients with AN to produce greater skin color reduction and melanin index reduction [10]. To objectively quantify the level of changes in pigmentation using reflectance spectrophotometry, other parameters such as texture and thickness were not considered for AN.
Our study uses an in-house assessment tool to help monitor the evolution of AN after treatment which was beneficial for skin texture assessment and pigmentation change. The result of the assessment tool aligns with the previous report [11]. Therefore, the ANSC is a valuable tool to validate the efficacy of treatment in changes.
In terms of the limitations of this study, long-term complications such as recurrence rate or long-term compliance or persistent improvements of treatment are not investigated. As our study included only the adolescent age group and Fitzpatrick phototypes IV and V, the results are generalizable to these groups only. In our study, there is no placebo arm used; the equal efficacy of both treatments may lead to the explanation that there is no statistically significant difference between the groups. In addition, another study on subjects has revealed that a combination of keratolytic chemical peels and mild keratolytic applications ensure promising results on skin improvement of AN [12]. Further studies may focus on different concentrations or combinations of other creams for increased effectiveness to reduce skin hyperpigmentation.
Conclusion
Both 10% salicylic acid and 10% urea creams are effective in reducing hyperpigmentation in AN, in which the 10% salicylic acid and 10% urea cream demonstrate similar efficacy and safety profiles.
Availability of data and material
Available upon request.
Code availability
Not applicable.
References
Phiske MM (2014) An approach to acanthosis nigricans. Indian Dermatol Online J 5(3):239–249
Das A, Datta D, Kassir M, Wollina U, Galadari H, Lotti T et al (2020) Acanthosis nigricans: a review. J Cosmet Dermatol 19(8):1857–1865
Treesirichod A, Chaithirayanon S, Chaikul T, Chansakulporn S (2021) The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J Dermatolog Treat. 32(7):837–842
Pan M, Heinecke G, Bernardo S, Tsui C, Levitt J (2013) Urea: a comprehensive review of the clinical literature. Dermatol Online J 19(11):20392
Arif T (2015) Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol 8:455–461
Treesirichod A, Chaithirayanon S, Wongjitrat N, Wattanapan P (2015) The efficacy of topical 0.1% adapalene gel for use in the treatment of childhood acanthosis nigricans: a pilot study. Indian J Dermatol. 60(1):103
Treesirichod A, Chuenboonngarm S, Kritsanaviparkporn C (2022) The efficacy and safety of 20% urea cream and 10% urea cream in the treatment of acanthosis nigricans in adolescents, a randomized comparative double-blind study. J Cosmet Dermatol 21(7):2859–2864
Walling HW, Messingham M, Myers LM, Mason CL, Strauss JS (2003) Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol 2(6):677–681
Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, Zamora-Gonzalez J, Cardoso-Saldana G, Jurado-Santacruz F et al (2006) Comparison of metformin versus rosiglitazone in patients with Acanthosis nigricans: a pilot study. J Drugs Dermatol 5(9):884–889
Leerapongnan P, Jurairattanaporn N, Kanokrungsee S, Udompataikul M (2020) Comparison of the effectiveness of fractional 1550-nm erbium fiber laser and 0.05% tretinoin cream in the treatment of acanthosis nigricans: a prospective, randomized, controlled trial. Lasers Med Sci. 35(5):1153–1158
Kritsanaviparkporn C, Treesirichod A (2022) Comparing the efficacy and safety profiles of 0.025% and 0.05% tretinoin creams in treating acanthosis nigricans: a randomized double-blinded study. Arch Dermatol Res. https://doi.org/10.1007/s00403-022-02472-7
Zeeshan M, Arfeen N, Sonthalia S, Singh A, Roy PK (2022) Treatment of acanthosis nigricans with sequential salicylic acid-mandelic acid combination peel and maintenance with glycolic acid-urea combination cream: A retrospective pilot study. J Cosmet Dermatol 21(9):3905–3909
Acknowledgements
We would like to acknowledge all participants and staff who take part in the study. This research is fundamentally supported by the Faculty of Medicine, Srinakharinwirot University, Thailand.
Funding
This study is funded by the Faculty of Medicine, Srinkharinwirot University, Thailand.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design, data collection, statistical analysis, manuscript preparation, and review.
Corresponding author
Ethics declarations
Conflicts of interests
There is no conflict of interest.
Ethics approval
The study has been approved by the Institutional Review Board and Ethics Committee of Srinakharinwirot University.
Consent to participate
All patients have provided written informed consent.
Consent for publication
Written informed consent has been provided by all patients before enrolment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
10% salicylic acid and 10% urea creams are efficacious and safe in the treatment of acanthosis nigricans.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Treesirichod, A., Thaneerat, N. & Kangvanskol, W. A comparison of the efficacy and safety profiles of 10% salicylic acid and 10% urea creams in treating acanthosis nigricans in adolescents: a randomized double-blinded study. Arch Dermatol Res 315, 2091–2097 (2023). https://doi.org/10.1007/s00403-023-02605-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-023-02605-6