Abstract
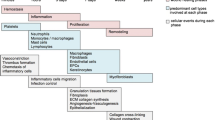
The migration and proliferation of keratinocytes is critical to wound re-epithelialization and defects in this function are associated with the clinical phenomenon of chronic non-healing wounds. Advanced glycation end products (AGEs) occur through non-enzymatic glycation of long-lived proteins in diabetes and play important roles in diabetic complications. However, specific roles for AGEs in keratinocyte migration and proliferation, and the underlying molecular mechanisms, have not been fully established. The aim of the current study was to elucidate the interaction between AGE-modified bovine serum albumin (AGE-BSA) and keratinocytes. As a result, we found that AGE-BSA had no effect on the viability of keratinocytes for up to 48 h of incubation with 50 μg/ml of AGE-BSA. AGE-BSA (but not non-glycated BSA) exerted a concentration-dependent suppression of keratinocyte migration at a range of concentrations. The expression of matrix metalloproteinase-9 (MMP-9) was significantly up-regulated in keratinocytes incubated with increasing AGE-BSA, but tissue inhibitor of metalloproteinases-1 (TIMP-1) expression was down-regulated. AGE-BSA also profoundly depressed phospho-focal adhesion kinase-Tyr397 (p-FAK) and α2β1 integrin expression, while total-FAK expression levels remained constant, in keratinocytes. The proliferative capacity of keratinocytes was diminished after 72 h AGE-BSA incubation. Taken together, these findings suggested that in the presence of AGE-BSA, keratinocytes lose their migratory and proliferation abilities. These data also indicated that, in the context of the chronic hyperglycemia in diabetes, the effects of AGE-BSA on keratinocyte migration might be mediated through MMP-9/TIMP-1, p-FAK and α2β1 integrin.









Similar content being viewed by others
References
Bhagavathula N, Nerusu KC, Lal A et al (2004) Rosiglitazone inhibits proliferation, motility, and matrix metalloproteinase production in keratinocytes. J Invest Dermatol 122:130–139
Boulton AJ, Vileikyte L, Ragnarson-Tennvall G et al (2005) The global burden of diabetic foot disease. Lancet 366:1719–1724
Brem H, Sheehan P, Boulton AJ (2004) Protocol for treatment of diabetic foot ulcers. Am J Surg 187:1S–10S
Chebassier N, El Houssein O, Viegas I et al (2004) In vitro induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in keratinocytes by boron and manganese. Exp Dermatol 13:484–490
Chen Q, Dong L, Wang L et al (2009) Advanced glycation end products impair function of late endothelial progenitor cells through effects on protein kinase akt and cyclooxygenase-2. Biochem Biophys Res Commun 381:192–197
Falanga V (2005) Wound healing and its impairment in the diabetic foot. Lancet 366:1736–1743
Gawlowski T, Stratmann B, Stirban AO et al (2007) Ages and methylglyoxal induce apoptosis and expression of mac-1 on neutrophils resulting in platelet-neutrophil aggregation. Thromb Res 121:117–126
Golubovskaya VM, Kweh FA, Cance WG (2009) Focal adhesion kinase and cancer. Histol Histopathol 24:503–510
Hattori N, Mochizuki S, Kishi K et al (2009) Mmp-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol 175:533–546
Jeffcoate W, Bakker K (2005) World diabetes day: footing the bill. Lancet 365:1527
Kim LT, Wu J, Bier-Laning C et al (2000) Focal adhesion kinase up-regulation and signaling in activated keratinocytes. J Surg Res 91:65–69
Kyriakides TR, Wulsin D, Skokos EA et al (2009) Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol 28:65–73
Li J, Chen J, Kirsner R (2007) Pathophysiology of acute wound healing. Clin Dermatol 25:9–18
Liu Y, Liang C, Liu X et al (2010) Ages increased migration and inflammatory responses of adventitial fibroblasts via rage, mapk and nf-kappab pathways. Atherosclerosis 208:34–42
Lobmann R, Schultz G, Lehnert H (2005) Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care 28:461–471
Lohwasser C, Neureiter D, Weigle B et al (2006) The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J Invest Dermatol 126:291–299
Mirastschijski U, Schnabel R, Claes J et al (2010) Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-β1-promoted myofibroblast formation and function. Wound Repair Regen 18:223–234
Morita K, Urabe K, Moroi Y et al (2005) Migration of keratinocytes is impaired on glycated collagen I. Wound Repair Regen 13:93–101
Nah SS, Choi IY, Yoo B et al (2007) Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and tnf-alpha in human osteoarthritic chondrocytes. FEBS Lett 581:1928–1932
Navaratna D, McGuire PG, Menicucci G et al (2007) Proteolytic degradation of ve-cadherin alters the blood-retinal barrier in diabetes. Diabetes 56:2380–2387
O’Toole EA (2001) Extracellular matrix and keratinocyte migration. Clin Exp Dermatol 26:525–530
O’Toole EA, van Koningsveld R, Chen M et al (2008) Hypoxia induces epidermal keratinocyte matrix metalloproteinase-9 secretion via the protein kinase c pathway. J Cell Physiol 214:47–55
Peppa M, Brem H, Ehrlich P et al (2003) Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes 52:2805–2813
Raja, Sivamani K, Garcia MS et al. (2007) Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front Biosci 12:2849–2868
Reiss MJ, Han YP, Garcia E et al (2010) Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery 147:295–302
Santoro MM, Gaudino G (2005) Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res 304:274–286
Scott KA, Arnott CH, Robinson SC et al (2004) Tnf-alpha regulates epithelial expression of mmp-9 and integrin alphavbeta6 during tumour promotion. A role for tnf-alpha in keratinocyte migration? Oncogene 23:6954–6966
Sieg DJ, Hauck CR, Schlaepfer DD (1999) Required role of focal adhesion kinase (fak) for integrin-stimulated cell migration. J Cell Sci 112(Pt 16):2677–2691
Singh N, Armstrong DG, Lipsky BA (2005) Preventing foot ulcers in patients with diabetes. JAMA 293:217–228
Singh R, Barden A, Mori T et al (2001) Advanced glycation end-products: a review. Diabetologia 44:129–146
Sohn H, Kim YS, Kim HT et al (2006) Ganglioside gm3 is involved in neuronal cell death. FASEB J 20:1248–1250
Teixeira AS, Caliari MV, Rocha OA et al (1999) Aminoguanidine prevents impaired healing and deficient angiogenesis in diabetic rats. Inflammation 23:569–581
Thompson CC, Ashcroft FJ, Patel S et al (2007) Pancreatic cancer cells overexpress gelsolin family capping proteins, which contribute to their cell motility. Gut 56:95–106
Thornalley PJ (1998) Cell activation by glycated proteins. Age receptors, receptor recognition factors and functional classification of ages. Cell Mol Biol (Noisy-le-grand) 44:1013–1023
Tokumaru S, Sayama K, Shirakata Y et al (2005) Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide ll-37. J Immunol 175:4662–4668
Valencia JV, Weldon SC, Quinn D et al (2004) Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem 324:68–78
Watson A, Morris VL, Chan BM (2009) Coordinated integrin and growth factor regulation of primary keratinocyte migration mediated through extracellular signal regulated kinase and phosphoinositide 3-kinase. Arch Dermatol Res 301:307–317
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83:835–870
Xu H, Shen J, Liu H et al (2006) Morroniside and loganin extracted from cornus officinalis have protective effects on rat mesangial cell proliferation exposed to advanced glycation end products by preventing oxidative stress. Can J Physiol Pharmacol 84:1267–1273
Yang C, Zhu P, Yan L et al (2009) Dynamic changes in matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 levels during wound healing in diabetic rats. J Am Podiatr Med Assoc 99:489–496
Yang W, Lu J, Weng J et al (2010) Prevalence of diabetes among men and women in china. N Engl J Med 362:1090–1101
Yoon SJ, Yoon YW, Lee BK et al (2009) Potential role of hmg coa reductase inhibitor on oxidative stress induced by advanced glycation endproducts in vascular smooth muscle cells of diabetic vasculopathy. Exp Mol Med 41:802–811
Yurko MA, O’Toole EA, Woodley DT (2001) Phosphorylation of focal adhesion kinase [pp125(fak)] is increased in human keratinocytes induced to migrate by extracellular matrices. J Cell Physiol 188:24–32
Zhang F, Kent KC, Yamanouchi D et al (2009) Anti-receptor for advanced glycation end products therapies as novel treatment for abdominal aortic aneurysm. Ann Surg 250:416–423
Zhang ZG, Bothe I, Hirche F et al (2006) Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: Contribution of α2β1 integrin. J Cell Sci 119:1886–1895
Acknowledgments
This research was supported by the National Science Foundation of China (Grant No. 81070660) and the Science and Technology Foundation of Guangdong province (Grant No. 0321). We are grateful to Shao-ling Zhang for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, P., Yang, C., Chen, LH. et al. Impairment of human keratinocyte mobility and proliferation by advanced glycation end products-modified BSA. Arch Dermatol Res 303, 339–350 (2011). https://doi.org/10.1007/s00403-010-1102-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-010-1102-z