Abstract
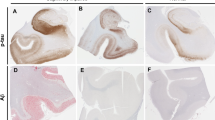
We investigate whether there is any association between the Braak neurofibrillary tangle (NFT) stage and clinical and MRI features in definite primary age-related tauopathy (PART). We analysed 52 cases with a Braak NFT tangle stage >0 and ≤IV, and a Thal phase of 0 (no beta-amyloid present). Twenty-nine (56%) were female. Median age at death was 88 years (IQR 82–92 years). Fifteen (29%) were TDP-positive (75% TDP stage I), 16 (31%) had argyrophilic grain disease and three (6%) had alpha-synuclein-positive Lewy bodies. TDP-43 inclusion when present were rare and predominantly perivascular. Of the 15 with TDP-43, three showed a moderate number of inclusions and also had hippocampal sclerosis, neuronal intranuclear inclusions and fine neurites of the CA1 region of the hippocampus. Four cases (8%) had an apolipoprotein epsilon 4 (APOE4) allele. There was a significant correlation between age at death and Braak NFT stage (r = 0.32, p = 0.02). After accounting for age at clinical examination, there were significant associations between Braak NFT stage, and WAIS-R Block Design and Trail Making Tests A and B, with higher Braak stage associated with poorer performances. Thirty of the 52 cases had completed an antemortem volumetric head MRI. Two separate MRI analyses revealed an association between higher Braak NFT stage and grey matter atrophy in the head of the left hippocampus. There were no significant clinical or radiologic associations with TDP-43. Findings from this study demonstrate that aggregated tau distribution is associated with poorer cognitive performance, as well as atrophy, in the absence of beta-amyloid. These findings support the parcellation of definite PART as a useful construct. The relatively low frequencies of APOE4, TDP-43, Lewy bodies, and hippocampal sclerosis, and the rarity and morphology of TDP-43 lesions are noted contrasts to what is typically observed in Alzheimer’s disease of the old.



Similar content being viewed by others
References
Adnan A, Barnett A, Moayedi M, McCormick C, Cohn M, McAndrews MP (2016) Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Struct Funct 221:2999–3012. doi:10.1007/s00429-015-1084-x
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445. doi:10.1002/ana.21154
Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, Ross OA, Dickson DW (2015) Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol 129:53–64. doi:10.1007/s00401-014-1358-z
Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H (2009) Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 117:125–136. doi:10.1007/s00401-008-0480-1
Aribisala BS, Royle NA, Maniega SM, Valdes Hernandez MC, Murray C, Penke L, Gow A, Starr JM, Bastin ME, Deary IJ et al (2014) Quantitative multi-modal MRI of the Hippocampus and cognitive ability in community-dwelling older subjects. Cortex 53:34–44. doi:10.1016/j.cortex.2013.12.012
Bancher C, Egensperger R, Kosel S, Jellinger K, Graeber MB (1997) Low prevalence of apolipoprotein E epsilon 4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol 94:403–409
Bancher C, Jellinger KA (1994) Neurofibrillary tangle predominant form of senile dementia of Alzheimer type: a rare subtype in very old subjects. Acta Neuropathol 88:565–570
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Del Tredici K (2014) Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta Neuropathol 128:767–772. doi:10.1007/s00401-014-1356-1
Chan D, Fox N, Rossor M (2002) Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology 58:838
Crary JF (2016) Primary age-related tauopathy and the amyloid cascade hypothesis: the exception that proves the rule? J Neurol Neuromed 1:53–57
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. doi:10.1007/s00401-014-1349-0
Crook R, Hardy J, Duff K (1994) Single-day apolipoprotein E genotyping. J Neurosci Methods 53:125–127
Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS et al (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA (1994) Hippocampal sclerosis: a common pathological feature of dementia in very old (> or =80 years of age) humans. Acta Neuropathol 88:212–221
Duyckaerts C, Braak H, Brion JP, Buee L, Del Tredici K, Goedert M, Halliday G, Neumann M, Spillantini MG, Tolnay M et al (2015) PART is part of Alzheimer disease. Acta Neuropathol 129:749–756. doi:10.1007/s00401-015-1390-7
Fahn S, Elton RL, Committee UD (1987) The unified Parkinson’s disease rating scale. Recent developments in Parkinson’s disease. MacMillian Healthcare Information, Florham Park, NJ, pp 153–163, 293–304
Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR et al (1998) Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci 18:1841–1847
Folstein MF, Robins LN, Helzer JE (1983) The mini-mental state examination. Arch Gen Psychiatry 40:812
Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM et al (2010) Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 67:1238–1250. doi:10.1001/archneurol.2010.254
Giaccone G (2015) The existence of primary age-related tauopathy suggests that not all the cases with early Braak stages of neurofibrillary pathology are Alzheimer’s disease. J Alzheimers Dis 48:919–921. doi:10.3233/JAD-150435
Gibb WR (1986) Idiopathic Parkinson’s disease and the Lewy body disorders. Neuropathol Appl Neurobiol 12:223–234
Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31:337–350. doi:10.1007/s10654-016-0149-3
Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V (2003) Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus 13:164–174. doi:10.1002/hipo.10064
Hatanpaa KJ, Bigio EH, Cairns NJ, Womack KB, Weintraub S, Morris JC, Foong C, Xiao G, Hladik C, Mantanona TY et al (2008) TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 67:271–279. doi:10.1097/NEN.0b013e31816a12a6
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hodges JR, Patterson K (2007) Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 6:1004–1014. doi:10.1016/S1474-4422(07)70266-1
Jack CR Jr, Knopman DS, Chetelat G, Dickson D, Fagan AM, Frisoni GB, Jagust W, Mormino EC, Petersen RC, Sperling RA et al (2016) Suspected non-Alzheimer disease pathophysiology–concept and controversy. Nat Rev Neurol 12:117–124. doi:10.1038/nrneurol.2015.251
James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA (2016) TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. doi:10.1093/brain/aww224
Jellinger K (2003) Plaque-predominant and tangle-predominant variants of Alzheimer’s disease. In: Dickson D (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, pp 66–68
Jellinger KA (1998) Dementia with grains (argyrophilic grain disease). Brain Pathol 8:377–386
Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm (Vienna) 111:1219–1235. doi:10.1007/s00702-004-0138-7
Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, Dickson DW, Hof PR, Hyman BT, Jack CR Jr et al (2015) PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol 129:757–762. doi:10.1007/s00401-015-1407-2
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117. doi:10.1007/s00401-006-0156-7
Jicha GA, Parisi JE, Dickson DW, Cha RH, Johnson KA, Smith GE, Boeve BF, Petersen RC, Knopman DS (2008) Age and apoE associations with complex pathologic features in Alzheimer’s disease. J Neurol Sci 273:34–39. doi:10.1016/j.jns.2008.06.008
Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127:441–450. doi:10.1007/s00401-013-1211-9
Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, Liesinger AM, Petersen RC, Parisi JE, Dickson DW (2016) Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 131:571–585. doi:10.1007/s00401-016-1537-1
Josephs KA, Stroh A, Dugger B, Dickson DW (2009) Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 118:349–358. doi:10.1007/s00401-009-0547-7
Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, Petrucelli L, Senjem ML, Ivnik RJ, Parisi JE et al (2015) TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol 78:697–709. doi:10.1002/ana.24493
Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF et al (2014) TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 127:811–824. doi:10.1007/s00401-014-1269-z
Kaplan E, Goodglass H, Weintraub S (1978) The Boston Naming Test. Veterans Administration Medical Center, Philadelphia, PA
Knopman DS, Jack CR Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM et al (2013) Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann Neurol 73:472–480. doi:10.1002/ana.23816
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE et al (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Kryscio RJ, Abner EL, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Lou W, Fardo DW, Cooper GE, Schmitt FA (2016) Self-reported memory complaints: a comparison of demented and unimpaired outcomes. J Prev Alzheimers Dis 3:13–19. doi:10.14283/jpad.2015.74
Lezak M (1995) Neuropsychological Assessment. Oxford University Press, New York, NY
Lin WL, Castanedes-Casey M, Dickson DW (2009) Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 68:1167–1176. doi:10.1097/NEN.0b013e3181baacec
Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4. doi:10.1007/s00401-009-0612-2
Martinez-Lage P, Munoz DG (1997) Prevalence and disease associations of argyrophilic grains of Braak. J Neuropathol Exp Neurol 56:157–164
Mattis S (1988) Dementia rating scale: professional manual. Psychological Assessment Resources, Odessa, FL
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2016) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. doi:10.1111/bpa.12424
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. doi:10.1212/01.wnl.0000187889.17253.b1
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796. doi:10.1016/S1474-4422(11)70156-9
Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, Rawal B, Parisi JE, Petersen RC, Kantarci K et al (2015) Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138:1370–1381. doi:10.1093/brain/awv050
Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, Smith CD, Fardo DW, Wang WX, Kryscio RJ et al (2016) “New Old Pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol 75:482–498. doi:10.1093/jnen/nlw033
Papp KV, Kaplan RF, Springate B, Moscufo N, Wakefield DB, Guttmann CR, Wolfson L (2014) Processing speed in normal aging: effects of white matter hyperintensities and hippocampal volume loss. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 21:197–213. doi:10.1080/13825585.2013.795513
Rey A (1964) L’examen clinique en psychologie. Presses Universitaires de France, Paris, France
Schacter DL, Wagner AD (1999) Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9:7–24. doi:10.1002/(SICI)1098-1063(1999)9:1<7:AID-HIPO2>3.0.CO;2-K
Spreen O, Strauss E (1998) Compendium of Neuropsychological tests, second edition: administration, norms and commentary. Oxford University Press, New York, NY
Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ (2005) Mayo’s older Americans normative studies: age- and IQ-adjusted norms for the Wechsler memory scale-revised. Clin Neuropsychol 19:378–463. doi:10.1080/13854040590945201
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, de Silva R, Lees A, Dickson DW (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Tsuang DW, Wilson RK, Lopez OL, Luedecking-Zimmer EK, Leverenz JB, DeKosky ST, Kamboh MI, Hamilton RL (2005) Genetic association between the APOE*4 allele and Lewy bodies in Alzheimer disease. Neurology 64:509–513. doi:10.1212/01.WNL.0000150892.81839.D1
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289. doi:10.1006/nimg.2001.0978
Ulrich J, Spillantini MG, Goedert M, Dukas L, Stähelin HB (1992) Abundant neurofibrillary tangles without senile plaques in a subset of patients with senile dementia. Neurodegeneration 1:257–284
Wechsler D (1987) Wechsler memory scale-revised (manual). Psychological Corporation, New York, NY
Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, Knopman DS, Boeve BF, Parisi JE, Petersen RC et al (2012) Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 11:868–877. doi:10.1016/S1474-4422(12)70200-4
WorkingG roup (1997) Consensus recommendation for the post-mortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for Neuropathologic Assessment of Alzheimer’s disease. Neurobiol Aging 18:S1–S2
Acknowledgements
This study was supported by the US National Institute of Aging (NIA) Grants R01 AG037491 (PI: KAJ), P50 AG16574 (PI: RCP), U01 AG006786 (PI: RCP), R01 AG11378 (PI: CRJ) and R01 AG041851 (PI: CRJ). We wish to thank Kris Johnson, Linda Rousseau, Virginia Phillips and Monica Casey-Castenedes for pathological support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Josephs, K.A., Murray, M.E., Tosakulwong, N. et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 133, 705–715 (2017). https://doi.org/10.1007/s00401-017-1681-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1681-2