Abstract
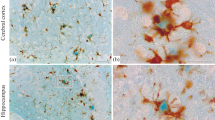
The routine diagnosis of argyrophilic grain disease is fraught by the lack of availability of an easily applied reproducible stain that can highlight the grain pathology with sensitivity and with minimal background. The Gallyas silver iodide technique is not widely used and, even in experienced hands, is difficult to perform due to inconsistencies inherent in silver-based techniques on thin sections. Grain pathology can be detected using immunohistochemistry for phosphorylated tau protein, but the grain pathology is most often masked by background tau-positive material; leading to problems with interpretation, especially for practitioners seeing small numbers of cases. There is a need for a reliable immunohistochemical stain that can detect grain pathology and provide a clear contrast between grains and other tau-positive neurodegenerative pathologies. We have investigated the novel ubiquitin-binding protein p62 as a potential biomarker for grain pathology in argyrophilic grain disease. Four cases of argyrophilic grain disease, in which the pathology was determined using the Gallyas silver iodide technique, were re-assessed using paraffin-embedded sections immunostained with antibodies specific for p62. We found that the detection of grain pathology was more sensitive than with silver-based techniques and that the resolution of the pathology was significantly improved. We suggest that p62 could be used to replace the Gallyas technique in the routine diagnosis of argyrophilic grain disease.

Similar content being viewed by others
References
Botez G, Schultz C, Ghebremedhin E, Bohl J, Braak E, Braak H (2000) Clinical aspects of argyrophilic grain disease. Nervenarzt 71:38–43
Braak H, Braak E (1987) Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer’s changes. Neurosci Lett 76:124–127
Braak H, Braak E (1998) Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm 105:801–819
Chan K-K, Lowe JS (2002) Techniques in neuropathology. In: Bancroft JD, Gamble M (eds) Theory and practice of histological techniques, 5th edn. Churchill Livingstone, London, pp 397–398
Chin SS-M, Goldman EJ (1996) Glial inclusions in CNS degenerative diseases, J. Neuropathol Exp Neurol 55:499–508
Ding ZT, Wang Y, Jiang YP, Yoshida M, Mimuro M, Inagaki T, Iwase T, Hashizume Y (2006) Argyrophilic grain disease: frequency and neuropathology in centenarians. Acta Neuropathol 111:320–328
Fujino Y, Wang DS, Thomas N, Espinoza M, Davies P, Dickson DW (2005) Increased frequency of argyrophilic grain disease in Alzheimer disease with 4R tau-specific immunohistochemistry. J Neuropathol Exp Neurol 64:209–214
Geetha T, Wooten MW (2002) Structure and functional properties of the ubiquitin binding protein p62. FEBS Lett 512(1–3):19–24
Kuusisto E, Salminen A, Alafuzof I (2001) Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 12(10):2085–2090
Martinez-Lage P, Munoz DG (1997) Prevalence and disease associations of argyrophilic grains of Braak. J Neuropathol Exp Neurol 56:157–164
Togo T, Dickson DW (2002) Ballooned neurons in progressive supranuclear palsy are usually due to concurrent argyrophilic grain disease, Acta Neuropathol 104:398–402
Togo T, Isojima D, Akatsu H, Suzuki K, Uchikado H, Katsuse O, Iseki E, Kosaka K, Hirayasu Y (2005) Clinical features of argyrophilic grain disease: a retrospective survey of cases with neuropsychiatric symptoms. Am J Geriatr Psychiatry 13(12):1083–1091
Tolnay A, Probst A (1998) Ballooned neurons expressing α,B-crystallin as a constant feature of the amygdala in argyrophilic grain disease. Neurosci Lett 246:165–168
Tolnay M, Ghebremedhin E, Probst A, Braak H (2003) Argyrophilic grain disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, ISN Neuropath Press, Basel, pp 132–136
Tolnay M, Probst A (1999) Review: Tau protein pathology in Alzheimer’s disease and related disorders. Neuropathol Appl Neurobiol 25:171–187
Tolnay M, Spillantini MG, Goedert M, Ulrich J, Langui D, Probst A (1997) Argyrophilic grain disease: widespread hyperphosphorylation of tau protein in limbic neurons, Acta Neuropathol 93:477–484
Uchihara T, Nakamura A, Mochizuki Y, Hayashi M, Orimo S, Isozaki E, Mizutani T (2005) Silver stainings distinguish Lewy bodies and glial cytoplasmic inclusions: comparison between Gallyas-Braak and Campbell-Switzer methods. Acta Neuropathol 110:255–260
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scott, I.S., Lowe, J.S. The ubiquitin-binding protein p62 identifies argyrophilic grain pathology with greater sensitivity than conventional silver stains. Acta Neuropathol 113, 417–420 (2007). https://doi.org/10.1007/s00401-006-0165-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0165-6