Abstract
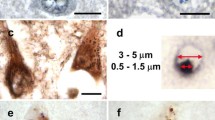
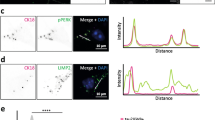
Protein misfolding is a distinguishing feature of a number of neurodegenerative diseases. Accumulation of misfolded protein often results in cellular lesions, the location of lesions correlating with the nature of symptoms. Alzheimer’s disease (AD), Progressive Supranuclear Palsy (PSP), Corticobasal Degeneration (CBD) and Pick’s Disease (PiD) all present with pathological lesions containing hyperphosphorylated filamentous tau protein; however, the location and type of lesion varies. In addition, granulovacuolar degeneration (GVD) bodies have been reported within hippocampal pyramidal neurons in AD, PSP, CBD and PiD tissue. GVDs are defined as electron-dense granules within double membrane-bound cytoplasmic vacuoles. We have previously reported that the phosphorylated form of stress-activated protein kinase/c-Jun N-terminal kinase (p-SAPK/JNK) accumulates in granules within hippocampal pyramidal cell bodies in AD tissue at the time that hyperphosphorylated tau begins to aggregate into early-stage NFTs. We now report that p-SAPK/JNK granules are found within the hippocampal CA1 region of PSP, CBD and PiD cases as well and that these granules are likely GVD bodies. Quantitatively, p-SAPK/JNK granules and GVDs are found in comparable numbers of CA1 cells. Within cells, p-SAPK/JNK granules are distributed throughout the cytoplasm in a manner similar to the distribution of GVDs and a subset of granules co-localize with GVD markers. Ultrastructurally, p-SAPK/JNK granules are located in large cytoplasmic vacuoles, thereby fitting the definition of a GVD body. With the implication of granular p-SAPK/JNK as a marker of GVDs, our study strongly suggests that a heterogeneous group of proteins form GVDs. The mechanism of GVD formation is therefore an interesting one, and is likely separate and distinct from the mechanism of tau inclusion formation.






Similar content being viewed by others
References
Arendt T (2004) Neurodegeneration and plasticity. Int J Dev Neurosci 22:507–514
Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol (Berl) 37:111–118
Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292:1552–1555
Bennett EJ, Bence NF, Jayakumar R, Kopito RR (2005) Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell 17:351–365
Berry RW, Quinn B, Johnson N, Cochran EJ, Ghoshal N, Binder LI (2001) Pathological glial tau accumulations in neurodegenerative disease: review and case report. Neurochem Int 39:469–479
Berry RW, Sweet AP, Clark FA, Lagalwar S, Lapin BR, Wang T, Topgi S, Guillozet-Bongaarts AL, Cochran EJ, Bigio EH, Binder LI (2004) Tau epitope display in progressive supranuclear palsy and corticobasal degeneration. J Neurocytol 33:287–295
Bondareff W, Wischik CM, Novak M, Roth M (1991) Sequestration of tau by granulovacuolar degeneration in alzheimer’s disease. Am J Pathol 139:641–647
Braak H, Braak E (1995) Staging of alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16: 271–278; discussion 278–284
Carmel G, Mager EM, Binder LI, Kuret J (1996) The structural basis of monoclonal antibody alz50’s selectivity for alzheimer’s disease pathology. J Biol Chem 271:32789–32795
Del Villar K, Miller CA (2004) Down-regulation of denn/madd, a tnf receptor binding protein, correlates with neuronal cell death in alzheimer’s disease brain and hippocampal neurons. Proc Natl Acad Sci USA 101:4210–4215
Dickson DW, Ksiezak-Reding H, Davies P, Yen SH (1987) A monoclonal antibody that recognizes a phosphorylated epitope in alzheimer neurofibrillary tangles, neurofilaments and tau proteins immunostains granulovacuolar degeneration. Acta Neuropathol (Berl) 73:254–258
Drexler HC (1997) Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci USA 94:855–860
Feany MB, Mattiace LA, Dickson DW (1996) Neuropathologic overlap of progressive supranuclear palsy, pick’s disease and corticobasal degeneration. J Neuropathol Exp Neurol 55:53–67
Flood F, Murphy S, Cowburn RF, Lannfelt L, Walker B, Johnston JA (2005) Proteasome-mediated effects on amyloid precursor protein processing at the gamma-secretase site. Biochem J 385:545–550
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in alzheimer’s disease. Proc Natl Acad Sci USA 100:10032–10037
Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW (1999) Involvement of caspases in proteolytic cleavage of alzheimer’s amyloid-beta precursor protein and amyloidogenic a beta peptide formation. Cell 97:395–406
Ghanevati M, Miller CA (2005) Phospho-beta-catenin accumulation in alzheimer’s disease and in aggresomes attributable to proteasome dysfunction. J Mol Neurosci 25:79–94
Ghoshal N, Smiley JF, DeMaggio AJ, Hoekstra MF, Cochran EJ, Binder LI, Kuret J (1999) A new molecular link between the fibrillar and granulovacuolar lesions of alzheimer’s disease. Am J Pathol 155:1163–1172
Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI (2002) Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and alzheimer’s disease. Exp Neurol 177:475–493
Ghribi O, Prammonjago P, Herman MM, Spaulding NK, Savory J (2003) Abeta(1-42)-induced jnk and erk activation in rabbit hippocampus is differentially regulated by lithium but is not involved in the phosphorylation of tau. Brain Res Mol Brain Res 119:201–206
Greenberg SG, Davies P, Schein JD, Binder LI (1992) Hydrofluoric acid-treated tau phf proteins display the same biochemical properties as normal tau. J Biol Chem 267:564–569
Herndon RM (1964) Lamellar bodies, an unusual arrangement of the granular endoplasmic reticulum. J Cell Biol 20:338–342
Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, Schlossman R, Podar K, Munshi NC, Mitsiades N, Anderson KC (2003) Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor ps-341. Blood 101:1530–1534
Holzer M, Gartner U, Stobe A, Hartig W, Gruschka H, Bruckner MK, Arendt T (2002) Inverse association of pin1 and tau accumulation in alzheimer’s disease hippocampus. Acta Neuropathol (Berl) 104:471–481
Ikegami K, Kimura T, Katsuragi S, Ono T, Yamamoto H, Miyamoto E, Miyakawa T (1996) Immunohistochemical examination of phosphorylated tau in granulovacuolar degeneration granules. Psychiatry Clin Neurosci 50:137–140
Ishizawa T, Sahara N, Ishiguro K, Kersh J, McGowan E, Lewis J, Hutton M, Dickson DW, Yen SH (2003) Co-localization of glycogen synthase kinase-3 with neurofibrillary tangles and granulovacuolar degeneration in transgenic mice. Am J Pathol 163:1057–1067
Jellinger KA, Stadelmann C (2000) Mechanisms of cell death in neurodegenerative disorders. J Neural Transm Suppl 59:95–114
Kannanayakal TJ, Tao H, Vandre DD, Kuret J (2006) Casein kinase-1 isoforms differentially associate with neurofibrillary and granulovacuolar degeneration lesions. Acta Neuropathol (Berl) 111:413–421
Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D, Van de Casteele M (2003) Amp-activated protein kinase can induce apoptosis of insulin-producing min6 cells through stimulation of c-jun-n-terminal kinase. J Mol Endocrinol 30:151–161
Keller JN, Hanni KB, Markesbery WR (2000) Impaired proteasome function in lzheimer’s disease. J Neurochem 75:436–439
King ME, Ghoshal N, Wall JS, Binder LI, Ksiezak-Reding H (2001) Structural analysis of pick’s disease-derived and in vitro-assembled tau filaments. Am J Pathol 158:1481–1490
Komori T (1999) Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and pick’s disease. Brain Pathol 9:663–679
Kuret J, Johnson GS, Cha D, Christenson ER, DeMaggio AJ, Hoekstra MF (1997) Casein kinase 1 is tightly associated with paired-helical filaments isolated from alzheimer’s disease brain. J Neurochem 69:2506–2515
Lagalwar S, Guillozet-Bongaarts AL, Berry RW, Binder LI (2006) Formation of phospho-sapk/jnk granules in the hippocampus is an early event in alzheimer’s disease. J Neuropathol Exp Neurol 65:455–464
Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion JP (2002) The active form of glycogen synthase kinase-3beta is associated with granulovacuolar degeneration in neurons in alzheimer’s disease. Acta Neuropathol (Berl) 103:91–99
Love S, Saitoh T, Quijada S, Cole GM, Terry RD (1988) Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, pick bodies and lewy bodies. J Neuropathol Exp Neurol 47:393–405
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in alzheimer disease and down syndrome. Proc Natl Acad Sci USA 82:4245–4249
Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial-Knecht E, Martinou JC, Arkinstall S (1997) Bcl-2 undergoes phosphorylation by c-jun n-terminal kinase/stress-activated protein kinases in the presence of the constitutively active gtp-binding protein rac1. J Biol Chem 272:25238–25242
Mena R, Robitaille Y, Cuello AC (1992) New patterns of intraneuronal accumulation of the microtubular binding domain of tau in granulovacuolar degeneration. J Geriatr Psychiatry Neurol 5:132–141
Mesulam MM (1999) Neuroplasticity failure in alzheimer’s disease: Bridging the gap between plaques and tangles. Neuron 24:521–529
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The consortium to establish a registry for alzheimer’s disease (cerad). Part ii. Standardization of the neuropathologic assessment of alzheimer’s disease. Neurology 41:479–486
Morroni M, Barbatelli G (2001) Stages of development of the ribosome-lamella complex: An ultrastructural study. Ultrastruct Pathol 25:335–343
Okamoto K, Hirai S, Iizuka T, Yanagisawa T, Watanabe M (1991) Reexamination of granulovacuolar degeneration. Acta Neuropathol (Berl) 82:340–345
Pasquini LA, Besio Moreno M, Adamo AM, Pasquini JM, Soto EF (2000) Lactacystin, a specific inhibitor of the proteasome, induces apoptosis and activates caspase-3 in cultured cerebellar granule cells. J Neurosci Res 59:601–611
Probst A, Herzig MC, Mistl C, Ipsen S, Tolnay M (2001) Perisomatic granules (non-plaque dystrophic dendrites) of hippocampal ca1 neurons in alzheimer’s disease and pick’s disease: a lesion distinct from granulovacuolar degeneration. Acta Neuropathol (Berl) 102:636–644
Qiu JH, Asai A, Chi S, Saito N, Hamada H, Kirino T (2000) Proteasome inhibitors induce cytochrome c-caspase-3-like protease-mediated apoptosis in cultured cortical neurons. J Neurosci 20:259–265
Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH (2000) Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: Differences in vitro between the mitogen-activated protein kinases erk2, c-jun n-terminal kinase and p38, and glycogen synthase kinase-3beta. J Neurochem 74:1587–1595
Rogatsky I, Logan SK, Garabedian MJ (1998) Antagonism of glucocorticoid receptor transcriptional activation by the c-jun n-terminal kinase. Proc Natl Acad Sci USA 95:2050–2055
Schwab C, DeMaggio AJ, Ghoshal N, Binder LI, Kuret J, McGeer PL (2000) Casein kinase 1 delta is associated with pathological accumulation of tau in several neurodegenerative diseases. Neurobiol Aging 21:503–510
Selznick LA, Holtzman DM, Han BH, Gokden M, Srinivasan AN, Johnson EM Jr., Roth KA (1999) In situ immunodetection of neuronal caspase-3 activation in alzheimer disease. J Neuropathol Exp Neurol 58:1020–1026
Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Bruck W, Jellinger K, Lassmann H (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in alzheimer’s disease. Evidence for apoptotic cell death. Am J Pathol 155:1459–1466
Su JH, Kesslak JP, Head E, Cotman CW (2002) Caspase-cleaved amyloid precursor protein and activated caspase-3 are co-localized in the granules of granulovacuolar degeneration in alzheimer’s disease and down’s syndrome brain. Acta Neuropathol (Berl) 104:1–6
Tomlinson BE, Kitchener D (1972) Granulovacuolar degeneration of hippocampal pyramidal cells. J Pathol 106:165–185
Trojanowski JQ, Schmidt ML, Shin RW, Bramblett GT, Rao D, Lee VM (1993) Altered tau and neurofilament proteins in neuro-degenerative diseases: Diagnostic implications for alzheimer’s disease and lewy body dementias. Brain Pathol 3:45–54
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the er to activation of jnk protein kinases by transmembrane protein kinase ire1. Science 287:664–666
Xu M, Shibayama H, Kobayashi H, Yamada K, Ishihara R, Zhao P, Takeuchi T, Yoshida K, Inagaki T, Nokura K (1992) Granulovacuolar degeneration in the hippocampal cortex of aging and demented patients—a quantitative study. Acta Neuropathol (Berl) 85:1–9
Yoshida H, Hastie CJ, McLauchlan H, Cohen P, Goedert M (2004) Phosphorylation of microtubule-associated protein tau by isoforms of c-jun n-terminal kinase (jnk). J Neurochem 90:352–358
Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA (2001) Activation and redistribution of c-jun n-terminal kinase/stress activated protein kinase in degenerating neurons in alzheimer’s disease. J Neurochem 76:435–441
Acknowledgments
The authors thank Dr. Angela Guillozet-Bongaarts (Northwestern University) for critical reading of the final versions of the manuscript and Dr. Akihiko Takashima and Dr. Naruhiko Sahara (RIKEN Institute) for help and advice with preliminary studies; Lennell Reynolds, Jr. from the Northwestern University Cell Imaging Facility for tissue embedding and ultrathin sectioning as well as for his expertise and advice with transmission electron microscopy; Dr. Eileen Bigio from the Northwestern University Cognitive Neurology and Alzheimer’s Disease Center and Dr. Dennis Dickson from the Mayo Clinic Alzheimer’s Disease Research Center for providing us with tissue; Dr. Merl F. Hoekstra from the ICOS Corporation for providing our laboratory with the anti-CKIδ and anti-CK1α antibodies; and Dr. Peter Davies from AECOM for allowing us to use the PHF-1 antibody. We are especially grateful to the patients and their families who have made this study possible. This work was supported by NIH grants AG09466 and AG021661.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lagalwar, S., Berry, R.W. & Binder, L.I. Relation of hippocampal phospho-SAPK/JNK granules in Alzheimer’s disease and tauopathies to granulovacuolar degeneration bodies. Acta Neuropathol 113, 63–73 (2007). https://doi.org/10.1007/s00401-006-0159-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0159-4