Abstract
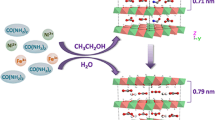
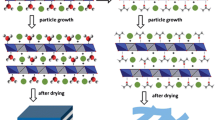
The goal of this study is to prepare hydrotalcite pellets and validate their potential utility in catalysts and catalysts support. Hydrotalcite pellets were synthesized by urea hydrolysis. Urea hydrolysis can provide both carbonate as the intercalated anion and hydroxyl anions to form Mg–Al layered double hydroxide (LDH) with carbonate intercalation. Urea hydrolysis was also used to generate NH3 which plays a critical role in the process of synthesis hydrotalcite pellets. Mechanism of the formation hydrotalcite pellets was also discussed. The as-prepared samples were well characterized by X-ray diffraction, scanning electron microscopy, transmission electronic microscope, N2 adsorption/desorption, and Fourier transform infrared spectroscopy, respectively. The results revealed that the hydrotalcite pellets were well-crystallized and formed by self-assembly of hexagonal platelets LDHs. The present work suggests that it is possible to grow hydrotalcite pellets directly through one-step aqueous solution-phase chemical route under controlled conditions.









Similar content being viewed by others
References
Choudary BM, Jaya VS, Reddy BR, Kantam ML, Rao MM, Madhavendra SS (2005) Chem Mater 17:2740–2743
Kim TW, Sahimi M, Tsotsis TT (2008) Ind Eng Chem Res 47:9127–9132
Winter F, Xia XY, Hereijgers BPC, Bitter JH, van Dillen AJ, Muhler M, de Jong KP (2006) J Phys Chem B 110:9211–9218
Motokura K, Nishimura D, Mori K, Mizugaki T, Ebitani K, Kaneda K (2004) J Am Chem Soc 126:5662–5663
Kantam ML, Laha S, Yadav J, Likhar PR, Sreedhar B, Jha S, Bhargava S, Udayakiran M, Jagadeesh B (2008) Org Lett 10:2979–2982
Kun R, Balázs M, Dékány I (2005) Coll Surf A 265:155–162
Meher LC, Gopinath R, Naik SN, Dalai AK (2009) Ind Eng Chem Res 48:1840–1846
Xiang X, Hima HI, Wang H, Li F (2008) Chem Mater 20:1173–1182
Gennequin C, Cousin R, Lamonier JF, Siffert S, Aboukais A (2008) Catal Commun 9:1639–1643
Yan K, Xie XM, Li JP, Wang XL, Wang ZZ (2007) J Nat Gas Chem 16:371–376
Ebitani K, Motokura K, Mori K, Mizugaki T, Kaneda K (2006) J Org Chem 71:5440–5447
Debecker DP, Gaigneaux EM, Busca G (2009) Chem Eur J 15:3920–3935
Ogawa M, Kaiho H (2002) Langmuir 18:4240–4242
Kloprogge JT, Hickey L, Trujillano R, Holgado M, San Roman MS, Rives V, Martens WN, Frost RL (2006) Cryst Growth Des 6:1533–1536
Naghash A, Etsell TH, Lu BJ (2008) Mater Chem 18:2562–2568
Zeng HY, Deng X, Wang YJ, Liao KB (2009) Aiche J 55:1229–1235
Turney (1995) United States Patent 5455058
Wang YC, Zhang FZ, Xu SL, Wang XY, Evans DG, Duan X (2008) Ind Eng Chem Res 47:5746–5750
Brei VV, Melezhyk OV, Starukh GM, Oranskaya EI, Mutovkin PA (2008) Micropor Mesopor Mat 113:411–417
Xi Y, Davis RJJ (2008) Catal 254:190–197
Zhao ZG, Geng FX, Bai JB, Cheng HM (2007) J Phys Chem C 111:3848–3852
Hornok V, Erd_helyi A, Dékány I (2005) Coll Polym Sci 283:1050–1055
Álvaro S, Enrique L (2009) Langmuir 25:3634–3639
Pu M, Wang YL, Liu LY, Liu YH, He J, Evans DG (2008) J Phys Chem Solids 69:1066–1069
Ogawa M, Asai S (2000) Chem Mater 12:3253–3255
Costantino U, Marmottini F, Nocchetti M, Vivani R (1998) Eur J Inorg Chem 10:1439–1446
Dékány I, Berger F, Imrik K, Lagaly G (1997) Coll Polym Sci 275:681–688
Benito P, Herrero M, Barriga C, Labajos FM, Rives V (2008) Inorg Chem 47:5453–5463
Acknowledgement
This work was supported by the Fundamental Research Funds of the Central University (HEUCF101010), the Key Technology R&D program of Heilongjiang Province (No.G202A423, No.TB06A05) and Science Fund for Young Scholar of Harbin City (No.2008RFQXG028).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Li, D., Yu, X. et al. Fabrication of layered double hydroxide spheres through urea hydrolysis and mechanisms involved in the formation. Colloid Polym Sci 288, 1411–1418 (2010). https://doi.org/10.1007/s00396-010-2271-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-010-2271-1