Abstract
Purpose
Metabolic changes during the perinatal period are known to promote obesity and type-2 diabetes in adulthood via perturbation of the microbiota. The risk factors for metabolic disorders include a high-fat diet (HFD) and exposure to pesticide residues. The objective of the present study was to evaluate the effects of perigestational exposure to a HFD and chlorpyrifos (CPF) on glycemia, lipid profiles, and microbial populations in Wistar dams and their female offspring. We also tested a preventive strategy based on treatment with the prebiotic inulin.
Methods
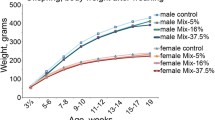
From 4 months before gestation to the end of the lactation period, six groups of dams were exposed to either a standard diet, a HFD alone, CPF alone, a combination of a HFD and CPF, and/or inulin supplementation. All female offspring were fed a standard diet from weaning to adulthood. We measured the impacts of these exposures on glycemia, the lipid profile, and the microbiota (composition, metabolite production, and translocation into tissues).
Results
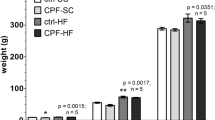
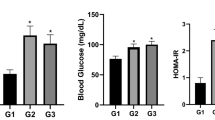
HFD exposure and CPF + HFD co-exposure induced dysmetabolism and an imbalance in the gut flora in both the dams and the female offspring. Inulin mitigated the impact of exposure to a HFD alone but not that of CPF + HFD co-exposure.
Conclusion
Our results provide a better understanding of the complex interactions between environmental pollutants and diet in early life, including in the context of metabolic diseases.




Similar content being viewed by others
Data availability
Data is available on demand by email to the corresponding author.
References
Seneviratne SN, Rajindrajith S (2022) Fetal programming of obesity and type 2 diabetes. World J Diabetes 13:482–497. https://doi.org/10.4239/wjd.v13.i7.482
Grandjean P, Barouki R, Bellinger DC et al (2015) Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology 156:3408–3415. https://doi.org/10.1210/EN.2015-1350
Mitanchez D, Chavatte-Palmer P (2018) Review shows that maternal obesity induces serious adverse neonatal effects and is associated with childhood obesity in their offspring. Acta Paediatr 107:1156–1165. https://doi.org/10.1111/apa.14269
Zhou L, Xiao X (2018) The role of gut microbiota in the effects of maternal obesity during pregnancy on offspring metabolism. Biosci Rep. https://doi.org/10.1042/BSR20171234
Mulligan CM, Friedman JE (2017) Maternal modifiers of the infant gut microbiota: metabolic consequences. J Endocrinol 235:R1–R12. https://doi.org/10.1530/JOE-17-0303
Stiemsma LT, Michels KB (2018) The role of the microbiome in the developmental origins of health and disease. Pediatrics 141:e20172437. https://doi.org/10.1542/peds.2017-2437
Tamburini S, Shen N, Wu HC, Clemente JC (2016) The microbiome in early life: implications for health outcomes. Nat Med 22:713–722. https://doi.org/10.1038/nm.4142
Efs EFSA, Medina-Pastor P, Triacchini G (2020) The 2018 European Union report on pesticide residues in food. EFSA J 18:e06057. https://doi.org/10.2903/j.efsa.2020.6057
Kaushal J, Khatri M, Arya SK (2021) A treatise on organophosphate pesticide pollution: current strategies and advancements in their environmental degradation and elimination. Ecotoxicol Environ Saf 207:111483. https://doi.org/10.1016/j.ecoenv.2020.111483
Dar MA, Kaushik G, Villarreal-Chiu JF (2019) Pollution status and bioremediation of chlorpyrifos in environmental matrices by the application of bacterial communities: a review. J Environ Manage 239:124–136. https://doi.org/10.1016/j.jenvman.2019.03.048
Rambabu L, Megson IL, Eddleston M (2020) Does oxidative stress contribute to toxicity in acute organophosphorus poisoning? – a systematic review of the evidence. Clin Toxicol 58:437–452. https://doi.org/10.1080/15563650.2019.1693589
Das S, Hageman KJ, Taylor M et al (2020) Fate of the organophosphate insecticide, chlorpyrifos, in leaves, soil, and air following application. Chemosphere 243:125194. https://doi.org/10.1016/j.chemosphere.2019.125194
Saunders M, Magnanti BL, Correia Carreira S et al (2012) Chlorpyrifos and neurodevelopmental effects: a literature review and expert elicitation on research and policy. Environ Health 11:S5. https://doi.org/10.1186/1476-069X-11-S1-S5
Joly Condette C, Elion Dzon B, Hamdad F et al (2016) Use of molecular typing to investigate bacterial translocation from the intestinal tract of chlorpyrifos-exposed rats. Gut Pathog 8:50. https://doi.org/10.1186/s13099-016-0129-x
Reygner J, Lichtenberger L, Elmhiri G et al (2016) Inulin supplementation lowered the metabolic defects of prolonged exposure to chlorpyrifos from gestation to young adult stage in offspring rats. PLoS One 11:e0164614. https://doi.org/10.1371/journal.pone.0164614
Liang Y, Zhan J, Liu D et al (2019) Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome 7:19. https://doi.org/10.1186/s40168-019-0635-4
Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD (2011) Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 7:639–646. https://doi.org/10.1038/nrendo.2011.126
Djekkoun N, Depeint F, Guibourdenche M et al (2022) Chronic perigestational exposure to chlorpyrifos induces perturbations in gut bacteria and glucose and lipid markers in female rats and their offspring. Toxics 10:138. https://doi.org/10.3390/toxics10030138
Joly Condette C, Khorsi-Cauet H, Morlière P et al (2014) Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS One 9:e102217. https://doi.org/10.1371/journal.pone.0102217
Fang B, Li JW, Zhang M et al (2018) Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem Toxicol 111:144–152. https://doi.org/10.1016/j.fct.2017.11.001
Elsharkawy EE, Yahia D, El-Nisr NA (2013) Sub-chronic exposure to chlorpyrifos induces hematological, metabolic disorders and oxidative stress in rat: Attenuation by glutathione. Environ Toxicol Pharmacol 35:218–227. https://doi.org/10.1016/j.etap.2012.12.009
Cotillard A, Kennedy SP, Kong LC et al (2013) Dietary intervention impact on gut microbial gene richness. Nature 500:585–588. https://doi.org/10.1038/nature12480
de Farias DP, de Araújo FF, Neri-Numa IA, Pastore GM (2019) Prebiotics: Trends in food, health and technological applications. Trends Food Sci Technol 93:23–35. https://doi.org/10.1016/j.tifs.2019.09.004
Akram W, Garud N, Joshi R (2019) Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov Ther 13:1–8. https://doi.org/10.5582/ddt.2019.01000
Brosseau C, Selle A, Palmer DJ et al (2019) Prebiotics: mechanisms and preventive effects in allergy. Nutrients 11:1841. https://doi.org/10.3390/nu11081841
Catry E, Bindels LB, Tailleux A et al (2018) Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 67:271–283. https://doi.org/10.1136/gutjnl-2016-313316
Guibourdenche M, Khayat El, el Sabbouri H, Bonnet F et al (2021) Perinatal exposure to chlorpyrifos and/or a high-fat diet is associated with liver damage in male rat offspring. Cells Dev 166:203678. https://doi.org/10.1016/j.cdev.2021.203678
Khayat El, El Sabbouri H, Gay-Ouéheillard J, Joumaa WH et al (2020) Does the perigestational exposure to chlorpyrifos and/or high-fat diet affect respiratory parameters and diaphragmatic muscle contractility in young rats? Food Chem Toxicol 140:111322. https://doi.org/10.1016/j.fct.2020.111322
Cochran RC, Kishiyama J, Aldous C et al (1995) Chlorpyrifos: Hazard assessment based on a review of the effects of short-term and long-term exposure in animals and humans. Food Chem Toxicol 33:165–172. https://doi.org/10.1016/0278-6915(94)00124-7
Condette CJ, Gay-Quéheillard J, Léké A et al (2013) Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the simulator of the human intestinal microbial ecosystem (SHIME) and in the rat. Environ Sci Pollut Res Int 20:2726–2734. https://doi.org/10.1007/s11356-012-1283-4
Reygner J, Joly Condette C, Bruneau A et al (2016) Changes in composition and function of human intestinal microbiota exposed to chlorpyrifos in oil as assessed by the SHIME® model. Int J Environ Res Public Health 13:1088. https://doi.org/10.3390/ijerph13111088
Joly Condette C, Bach V, Mayeur C et al (2015) Chlorpyrifos exposure during perinatal period affects intestinal microbiota associated with delay of maturation of digestive tract in rats. J Pediatr Gastroenterol Nutr 61:30–40. https://doi.org/10.1097/MPG.0000000000000734
Lecerf J-M, Dépeint F, Clerc E et al (2012) Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 108:1847–1858. https://doi.org/10.1017/S0007114511007252
Cortés-Álvarez NY, Vuelvas-Olmos CR, Pinto-González MF et al (2020) A high-fat diet during pregnancy impairs memory acquisition and increases leptin receptor expression in the hippocampus of rat offspring. Nutr Neurosci. https://doi.org/10.1080/1028415X.2020.1728473
Ribaroff GA, Wastnedge E, Drake AJ et al (2017) Animal models of maternal high fat diet exposure and effects on metabolism in offspring: a meta-regression analysis. Obes Rev 18:673–686. https://doi.org/10.1111/obr.12524
Tellechea ML, Mensegue MF, Pirola CJ (2017) The association between high fat diet around gestation and metabolic syndrome-related phenotypes in rats: a systematic review and meta-analysis. Sci Rep 7:5086. https://doi.org/10.1038/s41598-017-05344-7
Chu DM, Meyer KM, Prince AL, Aagaard KM (2016) Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 7:459–470. https://doi.org/10.1080/19490976.2016.1241357
Steegenga WT, Mischke M, Lute C et al (2017) Maternal exposure to a Western-style diet causes differences in intestinal microbiota composition and gene expression of suckling mouse pups. Mol Nutr Food Res 61:1600141. https://doi.org/10.1002/mnfr.201600141
Djekkoun N, Lalau J-D, Bach V et al (2021) Chronic oral exposure to pesticides and their consequences on metabolic regulation: role of the microbiota. Eur J Nutr. https://doi.org/10.1007/s00394-021-02548-6
Ruzzin J, Petersen R, Meugnier E et al (2010) Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect 118:465–471. https://doi.org/10.1289/ehp.0901321
Ibrahim MM, Fjære E, Lock E-J et al (2012) Metabolic impacts of high dietary exposure to persistent organic pollutants in mice. Toxicol Lett 215:8–15. https://doi.org/10.1016/j.toxlet.2012.09.022
Yanagisawa R, Koike E, Win-Shwe T-T et al (2014) Impaired lipid and glucose homeostasis in hexabromocyclododecane-exposed mice fed a high-fat diet. Environ Health Perspect 122:277–283. https://doi.org/10.1289/ehp.1307421
Adigun AA, Wrench N, Levin ED et al (2010) Neonatal parathion exposure and interactions with a high-fat diet in adulthood: adenylyl cyclase-mediated cell signaling in heart, liver and cerebellum. Brain Res Bull 81:605–612. https://doi.org/10.1016/j.brainresbull.2010.01.003
Sun Q, Qi W, Xiao X et al (2017) Imidacloprid promotes high fat diet-induced adiposity in female C57BL/6J mice and enhances adipogenesis in 3T3-L1 adipocytes via the AMPKα-mediated pathway. J Agric Food Chem 65:6572–6581. https://doi.org/10.1021/acs.jafc.7b02584
Sun Q, Xiao X, Kim Y et al (2016) imidacloprid promotes high fat diet-induced adiposity and insulin resistance in male C57BL/6J mice. J Agric Food Chem 64:9293–9306. https://doi.org/10.1021/acs.jafc.6b04322
Xiao X, Sun Q, Kim Y et al (2018) Exposure to permethrin promotes high fat diet-induced weight gain and insulin resistance in male C57BL/6J mice. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 111:405–416. https://doi.org/10.1016/j.fct.2017.11.047
Xiao X, Kim Y, Kim D et al (2017) Permethrin alters glucose metabolism in conjunction with high fat diet by potentiating insulin resistance and decreases voluntary activities in female C57BL/6J mice. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 108:161–170. https://doi.org/10.1016/j.fct.2017.07.053
La Merrill M, Karey E, Moshier E et al (2014) Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PLoS One 9:e103337. https://doi.org/10.1371/journal.pone.0103337
Yan J, Wang D, Meng Z et al (2020) Effects of incremental endosulfan sulfate exposure and high fat diet on lipid metabolism, glucose homeostasis and gut microbiota in mice. Environ Pollut. https://doi.org/10.1016/J.ENVPOL.2020.115697
Howell GE, Mulligan C, Meek E, Chambers JE (2015) Effect of chronic p, p′-dichlorodiphenyldichloroethylene (DDE) exposure on high fat diet-induced alterations in glucose and lipid metabolism in male C57BL/6H mice. Toxicology 328:112–122. https://doi.org/10.1016/j.tox.2014.12.017
Wang D, Yan S, Yan J et al (2019) Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis. Environ Pollut 246:630–638. https://doi.org/10.1016/j.envpol.2018.12.053
Choque Delgado GT, da Tamashiro WM, SC, (2018) Role of prebiotics in regulation of microbiota and prevention of obesity. Food Res Int 113:183–188. https://doi.org/10.1016/j.foodres.2018.07.013
Dewulf EM, Cani PD, Claus SP et al (2013) Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62:1112–1121. https://doi.org/10.1136/gutjnl-2012-303304
Hamaker BR, Tuncil YE (2014) A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 426:3838–3850. https://doi.org/10.1016/j.jmb.2014.07.028
Palou M, Sánchez J, García-Carrizo F et al (2015) Pectin supplementation in rats mitigates age-related impairment in insulin and leptin sensitivity independently of reducing food intake. Mol Nutr Food Res 59:2022–2033. https://doi.org/10.1002/mnfr.201500292
Singh A, Zapata RC, Pezeshki A et al (2018) Inulin fiber dose-dependently modulates energy balance, glucose tolerance, gut microbiota, hormones and diet preference in high-fat-fed male rats. J Nutr Biochem 59:142–152. https://doi.org/10.1016/j.jnutbio.2018.05.017
Weitkunat K, Stuhlmann C, Postel A et al (2017) Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci Rep 7:6109. https://doi.org/10.1038/s41598-017-06447-x
Zhang C, Yin A, Li H et al (2015) Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2:968–984. https://doi.org/10.1016/j.ebiom.2015.07.007
Ahmed W, Rashid S (2019) Functional and therapeutic potential of inulin: a comprehensive review. Crit Rev Food Sci Nutr 59:1–13. https://doi.org/10.1080/10408398.2017.1355775
Hiel S, Gianfrancesco MA, Rodriguez J et al (2020) Link between gut microbiota and health outcomes in inulin -treated obese patients: Lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr 39:3618–3628. https://doi.org/10.1016/j.clnu.2020.04.005
Shao T, Yu Q, Zhu T et al (2020) Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high-fat-diet-induced diabetes mice through the intestinal microflora improvement. Br J Nutr 123:308–318. https://doi.org/10.1017/S0007114519002332
Chen Y-T, Yang N-S, Lin Y-C et al (2018) A combination of Lactobacillus mali APS1 and dieting improved the efficacy of obesity treatment via manipulating gut microbiome in mice. Sci Rep 8:6153. https://doi.org/10.1038/s41598-018-23844-y
Gibson GR, Hutkins R, Sanders ME et al (2017) Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502. https://doi.org/10.1038/nrgastro.2017.75
Li L-L, Wang Y-T, Zhu L-M et al (2020) Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci Rep 10:978. https://doi.org/10.1038/s41598-020-58048-w
Moorthy M, Chaiyakunapruk N, Jacob SA, Palanisamy UD (2020) Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: a systematic review of randomised controlled trials. Trends Food Sci Technol 99:634–649. https://doi.org/10.1016/j.tifs.2020.03.036
Neyrinck AM, Bindels LB, Geurts L et al (2017) A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J Nutr Biochem 49:15–21. https://doi.org/10.1016/j.jnutbio.2017.07.008
Wang H, Wei C-X, Min L, Zhu L-Y (2018) Good or bad: gut bacteria in human health and diseases. Biotechnol Biotechnol Equip 32:1075–1080. https://doi.org/10.1080/13102818.2018.1481350
Zhao L, Zhang Q, Ma W et al (2017) A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct 8:4644–4656. https://doi.org/10.1039/C7FO01383C
Amar J, Chabo C, Waget A et al (2011) Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3:559–572. https://doi.org/10.1002/emmm.201100159
Cani PD, Possemiers S, Van de Wiele T et al (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103. https://doi.org/10.1136/gut.2008.165886
Rodes L, Saha S, Tomaro-Duchesneau C, Prakash S (2014) Microencapsulated Bifidobacterium longum subsp. infantis ATCC 15697 favorably modulates gut microbiota and reduces circulating endotoxins in F344 rats. BioMed Res Int. https://doi.org/10.1155/2014/602832
Acknowledgements
We thank Professor Jérôme Ausseil (Biochemistry Laboratory, Amiens University Medical Center, at the time of the work) for assistance with the metabolite assays. We also thank David Fraser PhD (Biotech Communication SARL, Ploudalmézeau, France) for copy-editing assistance.
Funding
The study was funded by the French Ministry of Higher Education, Research and the Hauts-de-France region via the 2017 “Fédératif Hospitalo-Universitaire 1000 Days for Health Proghomeo” program.
Author information
Authors and Affiliations
Contributions
Data curation: ND; investigation; ND, FD, MG, HEKES, AC, LR, JGQ, MB, AAS; formal analysis: ND, VB; visualization: ND, VB, HKC; methodology: ND, MG, HEKES, LR, LR, MB, AAS, HKC; writing—original draft; writing—review and editing: ND, FD, JDL, VB, HKC; validation: FD, HKC; supervision: JGQ, VB, HKC; funding acquisition: JGQ, HKC; conceptualization: HKC; project administration: HKC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported here.
Ethical approval
The study protocol was approved by the Regional Directorate for Health, Animal Protection and the Environment (Amiens, France) and the French Ministry of Research (reference: APAFIS # 8207-2016121322563594 v2, September 5th, 2017). All animals were treated in accordance with the European Union Directive 2010/63 and monitored daily for well-being.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Djekkoun, N., Depeint, F., Guibourdenche, M. et al. Perigestational exposure of a combination of a high-fat diet and pesticide impacts the metabolic and microbiotic status of dams and pups; a preventive strategy based on prebiotics. Eur J Nutr 62, 1253–1265 (2023). https://doi.org/10.1007/s00394-022-03063-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03063-y