Abstract
Purpose
Postmenopausal osteoporosis (PMO) is usually managed by conventional drug treatment. However, prolonged use of these drugs cause side effects. Gut microbiota may be a potential target for treatment of PMO. This work was a three-month intervention trial aiming to evaluate the added effect of probiotics as adjunctive treatment for PMO.
Methods
Forty patients with PMO were randomized into probiotic (n = 20; received Bifidobacterium animalis subsp. lactis Probio-M8 [Probio-M8], calcium, calcitriol) and placebo (n = 20; received placebo material, calcium, calcitriol) groups. The bone mineral density of patients was measured at month 0 (0 M; baseline) and month 3 (3 M; after three-month intervention). Blood and fecal samples were collected 0 M and 3 M. Only 15 and 12 patients from Probio-M8 and placebo groups, respectively, provided complete fecal samples for gut microbiota analysis.
Results
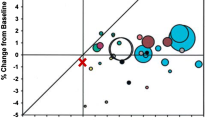
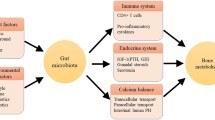
No significant change was observed in the bone mineral density of patients at 3 M. Co-administering Probio-M8 improved the bone metabolism, reflected by an increased vitamin D3 level and decreased PTH and procalcitonin levels in serum at 3 M. Fecal metagenomic analysis revealed modest changes in the gut microbiome in both groups at 3 M. Interestingly, Probio-M8 co-administration affected the gut microbial interactive correlation network, particularly the short-chain fatty acid-producing bacteria. Probio-M8 co-administration significantly increased genes encoding some carbohydrate metabolism pathways (including ABC transporters, the phosphotransferase system, and fructose and mannose metabolism) and a choline-phosphate cytidylyltransferase.
Conclusions
Co-administering Probio-M8 with conventional drugs/supplements was more efficacious than conventional drugs/supplements alone in managing PMO. Our study shed insights into the beneficial mechanism of probiotic adjunctive treatment.
Registration number of clinical trial
Chinese Clinical Trial Registry (identifier number: ChiCTR1800019268).




Similar content being viewed by others
Data availability
The sequence dataset was deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (accession number PRJNA773596).
References
Baccaro LF, Conde DM, Costa-Paiva L, Pinto-Neto AM (2015) The epidemiology and management of postmenopausal osteoporosis: a viewpoint from Brazil. Clin Interv Aging 10:583
Noh J-Y, Yang Y, Jung H (2020) Molecular mechanisms and emerging therapeutics for osteoporosis. Int J Mol Sci 21(20):7623
He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, Fu J, Lin X, Zheng G, Yu G (2020) Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY) 12(9):8583
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. The Lancet 377(9773):1276–1287
Seely KD, Kotelko CA, Douglas H, Bealer B, Brooks AE (2021) The Human gut microbiota: a key mediator of osteoporosis and osteogenesis. Int J Mol Sci 22(17):9452
Ringe JD (2020) Plain vitamin D or active vitamin D in the treatment of osteoporosis: where do we stand today? Arch Osteoporos 15(1):182. https://doi.org/10.1007/s11657-020-00842-0
Tilyard MW, Spears GFS, Thomson J, Dovey S (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326(6):357–362. https://doi.org/10.1056/nejm199202063260601
Janoušek J, Pilařová V, Macáková K, Nomura A, Veiga-Matos J, Silva DDD, Remião F, Saso L, Malá-Ládová K, Malý J, Nováková L, Mladěnka P (2022) Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Criti rev clin lab sci. https://doi.org/10.1080/10408363.2022.2070595
Rizzoli R, Biver E (2020) Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep 18(3):273–284
Ling C-w, Miao Z, Xiao M-l, Zhou H, Jiang Z, Fu Y, Xiong F, L-s-y Z, Liu Y-p, Wu Y-y (2021) The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab 106(10):e3852–e3864
Shen Q, Zhang C, Qin X, Zhang H, Zhang Z, Richel A (2021) Modulation of gut microbiota by chondroitin sulfate calcium complex during alleviation of osteoporosis in ovariectomized rats. Carbohyd Polym 266:118099
Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, Molloy C, O’Toole PW, Shanahan F, Molloy MG (2019) Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 58(12):2295–2304
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
Jia L, Tu Y, Jia X, Du Q, Zheng X, Yuan Q, Zheng L, Zhou X, Xu X (2021) Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif 54(7):e13075
Montazeri-Najafabady N, Ghasemi Y, Dabbaghmanesh MH, Talezadeh P, Koohpeyma F, Gholami A (2019) Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics antimicrob proteins 11(4):1145–1154
Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA (2017) Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr 36(7):497–506
Takimoto T, Hatanaka M, Hoshino T, Takara T, Tanaka K, Shimizu A, Morita H, Nakamura T (2018) Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized placebo-controlled double-blind clinical trial. Bioscience of Microbiota Food and Health. https://doi.org/10.12938/bmfh.18-006
Jansson P-A, Curiac D, Ahrén IL, Hansson F, Niskanen TM, Sjögren K, Ohlsson C (2019) Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol 1(3):e154–e162
Nilsson A, Sundh D, Bäckhed F, Lorentzon M (2018) Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med 284(3):307–317
Billington EO, Mahajan A, Benham JL, Raman M (2021) Effects of probiotics on bone mineral density and bone turnover: a systematic review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1998760
Zhang W, Wang Y, Li K, Kwok L-Y, Liu W, Zhang H (2020) Modulation of fatty acid metabolism improves oxygen tolerance of Bifidobacterium animalis ssp lactis Probio-M8. J Dairy Sci 103(10):8791–8795
Sun H, Zhao F, Liu Y, Ma T, Jin H, Quan K, Leng B, Zhao J, Yuan X, Li Z, Li F, Kwok L-Y, Zhang S, Sun Z, Zhang J, Zhang H (2022) Probiotics synergized with conventional regimen in managing Parkinson’s disease. npj Parkinson’s Disease. https://doi.org/10.1038/s41531-022-00327-6
Liu A, Ma T, Xu N, Jin H, Zhao F, Kwok L-Y, Zhang H, Zhang S, Sun Z (2021) Adjunctive probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome. Microbiol spectr 9(2):e00859-e1821
Narva M, Nevala R, Poussa T, Korpela R (2004) The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur J Nutr 43(2):61–68. https://doi.org/10.1007/s00394-004-0441-y
Dinan TG, Stanton C, Cryan JF (2013) Psychobiotics: a novel class of psychotropic. Biol Psychiat 74(10):720–726
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359
Kang DD, Li F, Kirton E, Thomas A, Egan R, An H, Wang Z (2019) MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7:e7359
Lee J, Jang JY, Kwon MS, Lim SK, Kim N, Lee J, Park HK, Yun M, Shin MY, Jo HE, Oh YJ, Ryu BH, Ko MY, Joo W, Choi HJ (2018) Mixture of two lactobacillus plantarum strains modulates the gut microbiota structure and regulatory t cell response in diet-induced obese mice. Mol Nutr Food Res 62(24):11. https://doi.org/10.1002/mnfr.201800329
Vasimuddin M, Misra S, Li H, Aluru S (2019) Efficient architecture-aware acceleration of BWA-MEM for multicore systems. 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS). Piscataway, IEEE, pp 314–324
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16):2078–2079
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25(7):1043–1055
Olm MR, Brown CT, Brooks B, Banfield JF (2017) dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J 11(12):2864–2868. https://doi.org/10.1038/ismej.2017.126
Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20(1):1–13
Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11(1):1–11
Becker KL, Snider R, Nylen ES (2010) Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol 159(2):253–264. https://doi.org/10.1111/j.1476-5381.2009.00433.x
Yerlikaya FH, Onmaz DE (2022) Inflammation and Bone turnover markers in adult obesity. J Clin Densitom. https://doi.org/10.1016/j.jocd.2022.08.002
Kärkkäinen MU, Lamberg-Allardt CJ, Ahonen S, Välimäki M (2001) Does it make a difference how and when you take your calcium? The acute effects of calcium on calcium and bone metabolism. Am J Clin Nutr 74(3):335–342
Narva M, Collin M, Lamberg-Allardt C, Kärkkäinen M, Poussa T, Vapaatalo H, Korpela R (2004) Effects of long-term intervention with Lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab 48(4):228–234
Bell TD, Demay MB, Burnett-Bowie SAM (2010) The biology and pathology of vitamin D control in bone. J Cell Biochem 111(1):7–13
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Heaney RP, Dowell MS, Hale CA, Bendich A (2003) Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22(2):142–146
Zaidi M, Moonga BS, Abe E (2002) Calcitonin and bone formation: a knockout full of surprises. J Clin Investig 110(12):1769–1771
Davey RA, Findlay DM (2013) Calcitonin: physiology or fantasy? J Bone Miner Res 28(5):973–979
Li J, Qiao H, LIN T, (2021) Diagnostic value of serum procalcitonin level combined with quantitative CT in elderly women with painful osteoporosis and its correlation with disease severity. Chin J Endocr Surg 6:189–192
MacDonald IJ, Tsai H-C, Chang A-C, Huang C-C, Yang S-F, Tang C-H (2021) Melatonin Inhibits osteoclastogenesis and osteolytic bone metastasis: implications for osteoporosis. Int J Mol Sci 22(17):9435
Kuo T-R, Chen C-H (2017) Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark res 5(1):1–9
Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR (2014) Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J cell physiol 229(11):1822–1830
Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei ANM, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R (2015) Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015:10. https://doi.org/10.1155/2015/897639
Singer FR, Eyre DR (2008) Using biochemical markers of bone turnover in clinical practice. Clevel Clin J Med 75(10):739–750
Liu ZY, Chen RQ, Jiang YT, Yang Y, He L, Luo CX, Dong JW, Rong LM (2019) A meta-analysis of serum osteocalcin level in postmenopausal osteoporotic women compared to controls. BMC Musculoskelet Disord 20(1):7. https://doi.org/10.1186/s12891-019-2863-y
Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, Coad J, Kruger MC (2021) The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR plus 5(3):e10452. https://doi.org/10.1002/jbm4.10452
Sun B, Ma T, Li Y, Yang N, Li B, Zhou X, Guo S, Zhang S, Kwok L-Y, Sun Z, Zhang H (2022) Bifidobacterium lactis probio-M8 adjuvant treatment confers added benefits to patients with coronary artery disease via target modulation of the gut-heart/-brain axes. Msystems. https://doi.org/10.1128/msystems.00100-22
Liu A, Ma T, Xu N, Jin H, Zhao F, Kwok L-Y, Zhang H, Zhang S, Sun Z (2021) Adjunctive probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome. Microbio spectr. https://doi.org/10.1128/Spectrum.00859-21
Xu HY, Ma C, Zhao FY, Chen P, Liu YH, Sun ZH, Cui LH, Kwok LY, Zhang HP (2021) Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur J Nutr 60(5):2553–2565. https://doi.org/10.1007/s00394-020-02437-4
Shanahan F, Hill C (2019) Language, numeracy and logic in microbiome science. Nat Rev Gastroenterol Hepatol 16(7):387–388. https://doi.org/10.1038/s41575-019-0163-5
Matchado MS, Lauber M, Reitmeier S, Kacprowski T, Baumbach J, Haller D, List M (2021) Network analysis methods for studying microbial communities: a mini review. Comput Struct Biotechnol J 19:2687–2698
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359(6380):1151–1156
Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, Chan FKL, Sung JJY, Yu J (2019) Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 68(4):654–662
Xu H, Zhao F, Hou Q, Huang W, Liu Y, Zhang H, Sun Z (2019) Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct 10(5):2618–2629
Kelder T, Stroeve JHM, Bijlsma S, Radonjic M, Roeselers G (2014) Correlation network analysis reveals relationships between diet-induced changes in human gut microbiota and metabolic health. Nutr Diabetes 4(6):e122–e122. https://doi.org/10.1038/nutd.2014.18
Ling CW, Miao ZL, Xiao ML, Zhou HW, Jiang ZL, Fu YQ, Xiong F, Zuo LSY, Liu YP, Wu YY, Jing LP, Dong HL, Chen GD, Ding D, Wang C, Zeng FF, Zhu HL, He Y, Zheng JS, Chen YM (2021) The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab 106(10):E3852–E3864. https://doi.org/10.1210/clinem/dgab492
Hayes CA, Dalia TN, Dalia AB (2017) Systematic genetic dissection of PTS in Vibrio cholerae uncovers a novel glucose transporter and a limited role for PTS during infection of a mammalian host. Mol Microbiol 104(4):568–579
Koropatkin NM, Cameron EA, Martens EC (2012) How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10(5):323–335
Morishita, (2009) Fluvastatin improves osteoporosis in fructose-fed insulin resistant model rats through blockade of the classical mevalonate pathway and antioxidant action. Int J Mol Med. https://doi.org/10.3892/ijmm_00000167
Mei ZD, Dong X, Qian Y, Hong D, Xie ZA, Yao GF, Qin A, Gao SY, Hu JY, Liang LM, Zheng Y, Su JC (2020) Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine 62:10. https://doi.org/10.1016/j.ebiom.2020.103111
Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL (2012) Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: The framingham osteoporosis study. J Bone Miner Res 27(5):1222–1230. https://doi.org/10.1002/jbmr.1581
Aleidi SM, Alnehmi EA, Alshaker M, Masood A, Benabdelkamel H, Al-Ansari MM, Rahman AMA (2021) A distinctive human metabolomics alteration associated with osteopenic and osteoporotic patients. Metabolites 11(9):14. https://doi.org/10.3390/metabo11090628
Funding
This research was supported by Inner Mongolia Science and Technology Major Projects (2021ZD0014) and the Earmarked Fund for CARS-36.
Author information
Authors and Affiliations
Contributions
JZ and HZ conceived and designed the trial. ZS supervised all bench work. ZG recruited patients, collected and summarized data. FZ, ZZ, KW, and YL analyzed the metagenomic sequencing data. FZ wrote the manuscript. LY Kwok reviewed and revised the manuscript critically. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
This intervention trial was approved by Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University (project number KY 2018010) and registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/; identifier number: ChiCTR1800019268). An informed consent was signed by all subjects prior to the study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, F., Guo, Z., Kwok, LY. et al. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur J Nutr 62, 965–976 (2023). https://doi.org/10.1007/s00394-022-03042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03042-3