Abstract
Purpose
Over-activation of the renin-angiotensin axis and worsening of vascular function are critical contributors to the development of hypertension. Therefore, inhibition of angiotensin-converting enzyme (ACE), a key factor of the renin-angiotensin axis, is a first line treatment of hypertension. Besides pharmaceutical ACE inhibitors, some natural peptides have been shown to exert ACE-inhibiting properties with antihypertensive effects and potentially beneficial effects on vascular function. In this study, the ACE-inhibiting potential and effects on vascular function of tryptophan-containing peptides were evaluated.
Methods
The ACE inhibitory action and stability of tryptophan-containing peptides was tested in endothelial cells—a major source of whole body ACE activity. Furthermore, the efficacy of peptides on vascular ACE activity, as well as vessel tone was assessed both ex vivo and in vivo.
Results
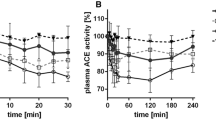
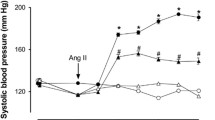
In human umbilical vein endothelial cells (HUVEC), isoleucine-tryptophan (IW) had the highest ACE inhibitory efficacy, followed by glutamic acid-tryptophan (EW) and tryptophan-leucine (WL). Whereas none of the peptides affected basal vessel tone (rat aorta), angiotensin I-induced vasoconstriction was blocked. IW effectively inhibited aortic ACE activity ex vivo taken from SHRs after 14-weeks of oral treatment with IW. Furthermore, IW treated SHRs showed better endothelium-dependent vessel relaxation compared to placebo.
Conclusion
This study shows strong ACE-inhibiting effects of IW, EW and WL in HUVECs and aorta. The peptides effectively counteract angiotensin-induced vasoconstriction and preserve endothelium-dependent vessel relaxation. Thus, tryptophan-containing peptides and particularly IW may serve as innovative food additives with the goal of protection from angiotensin II-induced worsening of vascular function.







Similar content being viewed by others
References
Foex P, Sear J (2004) Hypertension: pathophysiology and treatment. Contin Educ Anaesth Cirt Care Pain 4:71–75
Akpunonu BE, Mulrow PJ, Hoffman EA (1996) Secondary hypertension: evaluation and treatment. Dis Mon 42(10):609–722
Fanelli C, Zatz R (2011) Linking oxidative stress, the renin-angiotensin system and hypertension. Hypertension 57:373–374
Atlas SA (2007) The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 13(8 Suppl B):9–20
Brown NJ, Vaughan DE (1998) Angiotensin-converting enzyme inhibitors. Circulation 97:1411–1420
Ishiguro K, Hayashi K, Sasamura H, Sakamaki Y, Itoh H (2009) “Pulse” treatment with high-dose angiotensin blocker reverses renal arteriolar hypertrophy and regresses hypertension. Hypertension 53:83–89
Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau JL, Køber L, Aldo P, Maggioni MD, Solomon S, Swedberg K, Van de Werf F, White H et al (2003) Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 349:1893–1906
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ (2003) The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289:2560–2572
Collier S, Landram M (2012) Treatment of prehypertension: lifestyle and/or medication. Vasc Health Risk Manag 8:613–619. http://doi.org/10.2147/VHRM.S29138
Korhonen H, Pihlanto A (2006) Bioactive peptides: production and functionality. Int Dairy J 16:945–996
Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T (1995) Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci 78:777–783
Nakamura T, Mizutani J, Ohki K, Yamada K, Yamamoto N, Takeshi M, Takazawa K (2011) Casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro improves central blood pressure and arterial stiffness in hypertensive subjects: a randomized, double-blind, placebo-controlled trial. Atherosclerosis 219:298–303
Jäkälä P, Vapaatalo H (2010) Antihypertensive peptides from milk proteins. Pharmaceuticals 3:251–272
Lunow D, Kaiser S, Rückriemen J, Pohl C, Henle T (2015) Tryptophan-containing dipeptides are C-domain selective inhibitors of angiotensin converting enzyme. Food Chem 166:596–602
Wei L, Alhenc-Gelas F, Corvol P, Clauser E (1991) The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem 266:9002–9008
Elisseeva YE, Kugaevskaya EV (2009) Structure and physiological importance of angiotensin converting enzyme domains. Biomed Chem 3:237–247
Dicpinigaitis PV (2006) Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest 129:169–173
Khedr S, Martin M, Deussen A (2015) Inhibitory efficacy and biological variability of tryptophan containing dipeptides on human plasma angiotensin converting enzyme activity. J. Hypertens 4:2–6 (open access)
Gee NS, Kenny AJ (1987) Proteins of the kidney microvillar membrane. Enzymic and molecular properties of aminopeptidase W. Biochem J 246:97–102
Jaffe EA, Nachman RL, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745–2756
Zatschler B, Dieterich P, Müller B, Kasper M, Rauen U, Deussen A (2009) Improved vessel preservation after 4 days of cold storage: experimental study in rat arteries. J Vasc Surg 50:397–406. doi:10.1016/j.jvs.2009.04.064
Martin M, Kopaliani I, Jannasch A, Mund C, Todorov V, Henle T, Deussen A (2015) Antihypertensive and cardioprotective effects of the dipeptide isoleucine-tryptophan and whey protein hydrolysate. Acta Physiol 215:167–176. doi:10.1111/apha.12578
Bader M, Ganten D (2008) Update on tissue renin-angiotensin systems. J Mol Med 86(6):615–621. doi:10.1007/s00109-008-0336-0
Wu SJ, Soulez M, Yang YH, Chu CS, Shih SC, Hébert MJ, Kuo MC, Hsieh YJ (2015) Local augmented angiotensinogen secreted from apoptotic vascular endothelial cells is a vital mediator of vascular remodelling. PLoS One 10(7):e0132583. doi:10.1371/journal.phone.0132583
FitzGerald RJ, Murray BA, Walsh DJ (2004) Hypotensive peptides from milkproteins. J Nutr 134:980S–988 S
Kaiser S, Martin M, Lunow D, Rudolph S, Mertten S, Möckel U, Deussen A, Henle T (2016) Tryptophan-containing dipeptides are bioavailable and inhibit plasma human angiotensin-converting enzyme in vivo. Int Dairy J 52:107–114. http://doi.org/10.1016/j.idairyj.2015.09.004
Foltz M, Cerstiaens A, van Meensel A, Mols R, van der Pijl P.C, Duchateau G, Augustijns P. (2008) The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models. Peptides 29:1312–1320 (supplementary data)
Kopaliani I, Martin M, Zatschler B, Müller B, Deussen A (2016) Whey peptide Isoleucine-Tryptophan inhibits expression and activity of matrix metalloproteinase-2 in rat aorta. Peptides 82:52–59
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors declare that all animal studies have been approved by the appropriate ethics committee, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Khedr, S., Deussen, A., Kopaliani, I. et al. Effects of tryptophan-containing peptides on angiotensin-converting enzyme activity and vessel tone ex vivo and in vivo. Eur J Nutr 57, 907–915 (2018). https://doi.org/10.1007/s00394-016-1374-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1374-y