Abstract
Purpose
Lutein’s role on chronic hyperglycemia-induced oxidative stress and associated glucose homeostasis in heart and kidney is limited. Purpose of the study is to investigate the effect of lutein on cardiac and renal polyol pathway enzymes and oxidative stress markers under hyperglycemia-induced oxidative stress condition using streptozotocin (STZ)-injected rat model.
Methods
STZ-induced hyperglycemic (fasting blood glucose ≥11 mM) male Wistar rats were divided into two groups (n = 11/group). Group 1 received micellar lutein (39 nmol/day/rat) and group 2 (negative control) received micelle without lutein for 8 weeks. A separate group (no STZ injected) served as a positive control (n = 11/group). Oral glucose tolerance test (OGTT), biweekly urine glucose and activities of aldose reductase (AR) and sorbitol dehydrogenase (SDH) enzymes were assessed. Activities of antioxidant enzymes and antioxidant level were also evaluated.
Results
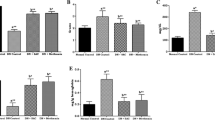
Lutein-administered hyperglycemic rats showed better glucose tolerance as evidenced with OGTT and biweekly urine glucose when compared to negative control. Activities of AR and SDH were decreased in heart and kidney of lutein-fed hyperglycemic rats. Also, they had significantly (p < 0.05) decreased malondialdehyde levels (66, 34, and 33 %) and increased reduced glutathione level (81, 18 and 92 %) in serum, heart and kidney, respectively. Altered antioxidant enzyme activities such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase and glutathione transferase were also affected in serum, heart and kidney of lutein-fed diabetic group.
Conclusion
Lutein prevented cardiac and renal injury in STZ-induced hyperglycemic rats due to potential amelioration of altered activities in polyol pathway and oxidative stress markers.







Similar content being viewed by others
References
Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J (2000) Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 26(3):163–176
Bantle JP (2009) Dietary fructose and metabolic syndrome and diabetes. J Nutr 139:1263S–1268S. doi:10.3945/jn.108.098020
Ceriello A (2000) Oxidative stress and glycemic regulation. Metabolism 49:27–29. doi:10.1016/S0026-0495(00)80082-7
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57:715S–724S
Johansen JS, Harris AK, Rychly DJ, Ergul A (2005) Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 4:5. doi:10.1186/1475-2840-4-5
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9. doi:10.2337/diabetes.48.1.1
Ying W (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10:179–206. doi:10.1089/ars.2007.1672
Chew BP, Brown CM, Park JS, Mixter PF (2003) Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res 23:3333–3339
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23:171–201. doi:10.1146/annurev.nutr.23.011702.073307
Nidhi B, Sharavana G, Ramaprasad TR, Vallikannan B (2015) Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopolysaccharide induced inflammation in rats. Food Funct. doi:10.1039/C4FO00606B
Hu B-J, Hu Y-N, Lin S, Ma W-J, Li X-R (2011) Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol 4:303–306. doi:10.3980/j.issn.2222-3959.2011.03.19
Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K et al (2010) Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 53:971–979. doi:10.1007/s00125-009-1655-6
Baskaran V, Sugawara T, Nagao A (2003) Phospholipids affect the intestinal absorption of carotenoids in mice. Lipids 38:705–711. doi:10.1007/s11745-003-1118-5
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V (2006) Enhanced lutein bioavailability by lyso-phosphatidylcholine in rats. Mol Cell Biochem 281:103–110. doi:10.1007/s11010-006-1337-3
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V (2007) Lutein and zeaxanthin in leafy greens and their bioavailability: olive oil influences the absorption of dietary lutein and its accumulation in adult rats. J Agric Food Chem 55:6395–6400. doi:10.1021/jf070482z
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V (2005) Determination of major carotenoids in a few Indian leafy vegetables by high-performance liquid chromatography. J Agric Food Chem 53:2838–2842. doi:10.1021/jf0481711
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Huggett ASG, Nixon DA (1957) Use of glucose oxidase, peroxidase and o-dianisidine in determination of blood and urinary glucose. Lancet 270(6991):368–370. doi:10.1016/S0140-6736(57)92595-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. doi:10.1016/0003-2697(79)90738-3
Owens CW, Belcher RV (1965) A colorimetric micro-method for the determination of glutathione. Biochem J 94:705–711. doi:10.1042/bj0940705
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226. doi:10.1016/0003-2697(76)90326-2
Driver AS, Kodavanti PRS, Mundy WR (2000) Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181. doi:10.1016/S0892-0362(99)00069-0
Kim HY, Oh JH (1999) Screening of Korean forest plants for rat lens aldose reductase inhibition. Biosci Biotechnol Biochem 63:184–188. doi:10.1271/bbb.63.184
Gerlach U, Hiby W (1974) Sorbitol dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3. Academic Press, New York, pp 569–573
Flohe L, Otting F (1984) Superoxide dismutase assays. Methods Enzymol 105:93–104
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 175:408–413. doi:10.1016/0003-2697(88)90564-7
Warholm M, Guthenberg C, von Bahr C, Mannervik B (1985) Glutathione transferases from human liver. Methods Enzymol 113:499–504
Low PA, Nickander KK, Tritschler HJ (1997) The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes 46:S38–S42. doi:10.2337/diab.46.2.S38
Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847. doi:10.1161/01.CIR.97.18.1837
Vincent HK, Bourguignon CM, Weltman AL, Vincent KR, Barrett E, Innes KE et al (2009) Effects of antioxidant supplementation on insulin sensitivity, endothelial adhesion molecules, and oxidative stress in normal-weight and overweight young adults. Metabolism 58:254–262. doi:10.1016/j.metabol.2008.09.022
Evans JL, Maddux BA, Goldfine ID (2005) The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 7:1040–1052. doi:10.1089/ars.2005.7.1040
Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee D-H (2007) Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the coronary artery risk development in young adults (CARDIA)/young adult longitudinal trends in antioxidants (YALTA). Clin Chem 53:447–455. doi:10.1373/clinchem.2006.074930
Faulks RM, Southon S (2005) Challenges to understanding and measuring carotenoid bioavailability. Biochim Biophys Acta 1740:95–100. doi:10.1016/j.bbadis.2004.11.012
Mogensen CE (1987) Early diabetic renal involvement and nephropathy. The diabetes annual, vol 3, pp 306–324
Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB (2005) Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Investig Ophthalmol Vis Sci 46:2092. doi:10.1167/iovs.04-1304
Romero MJ, Yao L, Sridhar S, Bhatta A, Dou H, Ramesh G et al (2013) L-citrulline protects from kidney damage in type 1 diabetic mice. Front Immunol 4:480. doi:10.3389/fimmu.2013.00480
Kumar V, Ahmed D, Gupta PS, Anwar F, Mujeeb M (2013) Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC Complement Altern Med 13:222. doi:10.1186/1472-6882-13-222
Muruganandan S, Gupta S, Kataria M, Lal J, Gupta P (2002) Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology 176:165–173. doi:10.1016/S0300-483X(02)00069-0
Mohamed AK, Bierhaus A, Schiekofer S, Tritschler H, Ziegler R, Nawroth PP (1999) The role of oxidative stress and NF-κB activation in late diabetic complications. BioFactors 10:157–167. doi:10.1002/biof.5520100211
Costagliola C, Iuliano G, Menzione M, Rinaldi E, Vito P, Auricchio G (1986) Effect of vitamin E on glutathione content in red blood cells, aqueous humor and lens of humans and other species. Exp Eye Res 43:905–914. doi:10.1016/0014-4835(86)90069-2
Acknowledgments
The authors are grateful to Dr. Mahenderkar, ex-chief Editor, Journal of Food Science and Technology, for editing the manuscript with respect to English language. This work was financially supported by the Major Laboratory Project, CSIR-Central Food Technological Research Institute, Mysore, India. Gurunathan Sharavana acknowledges the award of Senior Research Fellowship by the Department of Biotechnology, Government of India, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharavana, G., Joseph, G.S. & Baskaran, V. Lutein attenuates oxidative stress markers and ameliorates glucose homeostasis through polyol pathway in heart and kidney of STZ-induced hyperglycemic rat model. Eur J Nutr 56, 2475–2485 (2017). https://doi.org/10.1007/s00394-016-1283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1283-0