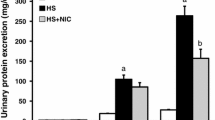
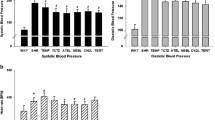
Abstract
Purpose
Arterial hypertension is associated with a high production of reactive oxygen species and a decrease in the antioxidant defense systems. Based on the lack of toxicity of l-carnitine (LC) and previous studies reporting beneficial effects of this compound in experimental models of hypertension, the aim of this work was to test the hypothesis that LC might protect the kidney against hypertension-induced oxidative damage, as well as to investigate the mechanisms involved in this effect. To this end, specific activities and protein/mRNA expression of the antioxidant enzymes (glutathione peroxidase, glutathione reductase, and superoxide dismutase), and those of NADPH oxidase (the main responsible for superoxide anion production in renal tissue) have been measured in renal cortex homogenates from NG-nitro-l-arginine methyl ester (L-NAME)-treated rats and control normotensive rats. In addition, components of the renin–angiotensin system (RAS) and redox-sensitive transcription factors (NF-κB, Nrf2, and PPARα) have also been evaluated.
Methods
Male Wistar rats aged 6–8 weeks were divided into four groups of six animals each: (1) control, normotensive Wistar rats (with free access to tap water); (2) Wistar rats subjected to treatment with 25 mg of L-NAME/kg body weight/day dissolved in the drinking water, in order to develop L-NAME-induced hypertension; (3) Wistar rats subjected to treatment with 400 mg of LC/kg body weight/day (also dissolved in the drinking water); and (4) L-NAME-treated rats subjected to simultaneous treatment with LC at the indicated doses.
Results
The beneficial effect of LC supplementation on oxidative damage in the renal cortex of hypertensive rats reversed hypertension-associated renal function damage and produced an upregulation of both antioxidant enzymes and eNOS, and with a downregulation of both NADPH oxidase and RAS components. LC improves the oxidative stress response through a specific modulation of NF-κB, Nrf2, and PPARα transcription factors. Thus, the low production of superoxide anions, subsequent to NADPH oxidase inhibition, might act by increasing the expression of Nrf2 and PPARα and by decreasing that of NF-κB, which, in turn, would enhance the antioxidant defense systems.
Conclusions
Our results might support the use of LC to prevent hypertension-induced renal damage.






Similar content being viewed by others
References
Touyz RM (2004) Reactive oxygen species and angiotensin II signaling in vascular cells-implications in cardiovascular disease. Braz J Med Biol Res 37:1263–1273
Manning RD Jr, Tian N, Meng S (2005) Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 25:311–317
Gomez-Amores L, Mate A, Miguel-Carrasco JL, Jimenez L, Jos A, Camean AM, Revilla E, Santa-Maria C, Vazquez CM (2007) l-Carnitine attenuates oxidative stress in hypertensive rats. J Nutr Biochem 18:533–540
Mate A, Miguel-Carrasco JL, Monserrat MT, Vazquez CM (2010) Systemic antioxidant properties of l-carnitine in two different models of arterial hypertension. J Physiol Biochem 66:127–136
Briones AM, Touyz RM (2010) Oxidative stress and hypertension: current concepts. Curr Hypertens Rep 12:135–142
Sedeek M, Hebert RL, Kennedy CR, Burns KD, Touyz RM (2009) Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens 18:122–127
Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J (2008) Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int 74(Suppl 111):S4–S9
Rodriguez-Iturbe B, Zhan CD, Quiroz Y, Sindhu RK, Vaziri ND (2003) Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension 41:341–346
Garrido AM, Griendling KK (2009) NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol 302:148–158
Zicha J, Dobesova Z, Kunes J (2006) Antihypertensive mechanisms of chronic captopril or N-acetylcysteine treatment in L-NAME hypertensive rats. Hypertens Res 29:1021–1027
Cediel E, Sanz-Rosa D, Oubina MP, de las Heras N, Gonzalez Pacheco FR, Vegazo O, Jimenez J, Cachofeiro V, Lahera V (2003) Effect of AT1 receptor blockade on hepatic redox status in SHR: possible relevance for endothelial function? Am J Physiol Regul Integr Comp Physiol 285:R674–R681
Kawai T, Masaki T, Doi S, Arakawa T, Yokoyama Y, Doi T, Kohno N, Yorioka N (2009) PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab Invest 89:47–58
Mate A, Miguel-Carrasco JL, Vazquez CM (2010) The therapeutic prospects of using l-carnitine to manage hypertension-related organ damage. Drug Discov Today 15:484–492
Arduini A, Bonomini M, Savica V, Amato A, Zammit V (2008) Carnitine in metabolic disease: potential for pharmacological intervention. Pharmacol Ther 120:149–156
Emami Naini A, Moradi M, Mortazavi M, Amini Harandi A, Hadizadeh M, Shirani F, Basir Ghafoori H, Emami Naini P (2012) Effects of oral l-carnitine supplementation on lipid profile, anemia, and quality of life in chronic renal disease patients under hemodialysis: a randomized, double-blinded, placebo-controlled trial. J Nutr Metab 2012(510483):1–6. doi:10.1155/2012/510483
Fatouros IG, Douroudos I, Panagoutsos S, Pasadakis P, Nikolaidis MG, Chatzinikolaou A, Sovatzidis A, Michailidis Y, Jamurtas AZ, Mandalidis D, Taxildaris K, Vargemezis V (2010) Effects of l-carnitine on oxidative stress responses in patients with renal disease. Med Sci Sports Exerc 42:1809–1818
Ruggenenti P, Cattaneo D, Loriga G, Ledda F, Motterlini N, Gherardi G, Orisio S, Remuzzi G (2009) Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: effects of acetyl-l-carnitine therapy. Hypertension 54:567–574
Miguel-Carrasco JL, Monserrat MT, Mate A, Vazquez CM (2010) Comparative effects of captopril and l-carnitine on blood pressure and antioxidant enzyme gene expression in the heart of spontaneously hypertensive rats. Eur J Pharmacol 632:65–72
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Gómez-Amores L, Mate A, Vázquez CM (2003) l-Carnitine transport in kidney of normotensive, Wistar-Kyoto rats: effect of chronic l-carnitine administration. Pharm Res 20:1133–1140
Marzinzig M, Nussler AK, Stadler J, Marzinzig E, Barthlen W, Nussler NC, Beger HG, Morris SM Jr, Bruckner UB (1997) Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide 1:177–189
Jentzsch AM, Bachmann H, Furst P, Biesalski HK (1996) Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 20:251–256
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Arthur JR, Boyne R (1985) Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci 36:1569–1575
Zalba G, Beaumont FJ, San Jose G, Fortuño A, Fortuño MA, Etayo JC, Diez J (2000) Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 35:1055–1061
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Chaves FJ, Mansego ML, Blesa S, Gonzalez-Albert V, Jimenez J, Tormos MC, Espinosa O, Giner V, Iradi A, Saez G, Redon J (2007) Inadequate cytoplasmic antioxidant enzymes response contributes to the oxidative stress in human hypertension. Am J Hypertens 20:62–69
Gomez-Amores L, Mate A, Revilla E, Santa-Maria C, Vazquez CM (2006) Antioxidant activity of propionyl-l-carnitine in liver and heart of spontaneously hypertensive rats. Life Sci 78:1945–1952
Duarte J, Jimenez R, O’Valle F, Galisteo M, Perez-Palencia R, Vargas F, Perez-Vizcaino F, Zarzuelo A, Tamargo J (2002) Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J Hypertens 20:1843–1854
Pedro-Botet J, Covas MI, Martin S, Rubies-Prat J (2000) Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens 14:343–345
Saez GT, Tormos C, Giner V, Chaves J, Lozano JV, Iradi A, Redon J (2004) Factors related to the impact of antihypertensive treatment in antioxidant activities and oxidative stress by-products in human hypertension. Am J Hypertens 17:809–816
Simic DV, Mimic-Oka J, Pljesa-Ercegovac M, Savic-Radojevic A, Opacic M, Matic D, Ivanovic B, Simic T (2006) Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J Hum Hypertens 20:149–155
Annadurai T, Vigneshwari S, Thirukumaran R, Thomas PA, Geraldine P (2011) Acetyl-l-carnitine prevents carbon tetrachloride-induced oxidative stress in various tissues of Wistar rats. J Physiol Biochem 67:519–530
Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L (2010) l-Carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept 161:58–66
Paravicini TM, Touyz RM (2008) NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31(Suppl 2):S170–S180
Zalba G, San Jose G, Moreno MU, Fortuno MA, Fortuno A, Beaumont FJ, Diez J (2001) Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension 38:1395–1399
Fortuño A, Olivan S, Beloqui O, San Jose G, Moreno MU, Diez J, Zalba G (2004) Association of increased phagocytic NADPH oxidase-dependent superoxide production with diminished nitric oxide generation in essential hypertension. J Hypertens 22:2169–2175
Moreno MU, San Jose G, Fortuño A, Beloqui O, Redon J, Chaves FJ, Corella D, Diez J, Zalba G (2007) A novel CYBA variant, the −675A/T polymorphism, is associated with essential hypertension. J Hypertens 25:1620–1626
Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285:R277–R297
Mister M, Noris M, Szymczuk J, Azzollini N, Aiello S, Abbate M, Trochimowicz L, Gagliardini E, Arduini A, Perico N, Remuzzi G (2002) Propionyl-l-carnitine prevents renal function deterioration due to ischemia/reperfusion. Kidney Int 61:1064–1078
Stasi MA, Scioli MG, Arcuri G, Mattera GG, Lombardo K, Marcellini M, Riccioni T, De Falco S, Pisano C, Spagnoli LG, Borsini F, Orlandi A (2010) Propionyl-l-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production. Arterioscler Thromb Vasc Biol 30:426–435
Surh YJ, Kundu JK, Na HK, Lee JS (2005) Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr 135:2993S–3001S
Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E (2008) Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res 33:2444–2471
Pendyala S, Natarajan V (2010) Redox regulation of NOX proteins. Respir Physiol Neurobiol 174:265–271
Chen HH, Sue YM, Chen CH, Hsu YH, Hou CC, Cheng CY, Lin SL, Tsai WL, Chen TW, Chen TH (2009) Peroxisome proliferator-activated receptor alpha plays a crucial role in l-carnitine anti-apoptosis effect in renal tubular cells. Nephrol Dial Transplant 24:3042–3049
Chao HH, Liu JC, Hong HJ, Lin JW, Chen CH, Cheng TH (2011) l-Carnitine reduces doxorubicin-induced apoptosis through a prostacyclin-mediated pathway in neonatal rat cardiomyocytes. Int J Cardiol 146:145–152
Jing L, Zhou LJ, Li WM, Zhang FM, Yuan L, Li S, Song J, Sang Y (2011) Carnitine regulates myocardial metabolism by Peroxisome Proliferator-Activated Receptor-alpha (PPARalpha) in alcoholic cardiomyopathy. Med Sci Monit 17:BR1–BR9
San Jose G, Fortuño A, Moreno MU, Robador PA, Bidegain J, Varo N, Beloqui O, Diez J, Zalba G (2009) The angiotensin-converting enzyme insertion/deletion polymorphism is associated with phagocytic NADPH oxidase-dependent superoxide generation: potential implication in hypertension. Clin Sci (Lond) 116:233–240
Rajasekar P, Viswanathan P, Anuradha CV (2008) Renoprotective action of l-carnitine in fructose-induced metabolic syndrome. Diabetes Obes Metab 10:171–180
Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, Vazquez CM (2008) The role of inflammatory markers in the cardioprotective effect of l-carnitine in L-NAME-induced hypertension. Am J Hypertens 21:1231–1237
Acknowledgments
This study was supported by Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación, PN de I+D+I 2008-2011 (PS09/01395) and Consejería de Salud, Junta de Andalucía (PI-0034). S. Zambrano, J.L. Miguel-Carrasco, and A. Blanca were supported by the grants from Consejería de Salud, Junta de Andalucía (PI-0034), and Instituto de Salud Carlos III (FIS PS09/01395). We thank the University of Seville’s Centro de Investigación, Tecnología e Innovación (CITIUS) for its technical assistance. We are also grateful to Dra. A. Fortuño for her help with the determination of NADPH oxidase activity.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zambrano, S., Blanca, A.J., Ruiz-Armenta, M.V. et al. The renoprotective effect of l-carnitine in hypertensive rats is mediated by modulation of oxidative stress-related gene expression. Eur J Nutr 52, 1649–1659 (2013). https://doi.org/10.1007/s00394-012-0470-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0470-x