Abstract
Background
Activated leukocytes may contribute to the development and progression of heart failure (HF). We investigated the predictive value of circulating levels of stable and readily detectable markers reflecting both monocyte/macrophage and T-cell activity, on clinical outcomes in HF patients with reduced ejection fraction (HFrEF).
Methods
The association between baseline plasma levels of soluble CD163 (sCD163), macrophage migration inhibitory factor (MIF), granulysin, soluble interleukin-2 receptor (sIL-2R), and activated leukocyte cell adhesion molecule (ALCAM) and the primary endpoint of death from any cause or first hospitalization for worsening of HF was evaluated using multivariable Cox proportional hazard models in 1541 patients with systolic HF and mild to moderate anemia, enrolled in the Reduction of Events by darbepoetin alfa in Heart Failure (RED-HF) trial. Modifying effects and interaction with darbepoetin alfa treatment were also assessed.
Results
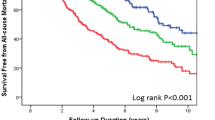
All leukocyte markers, except granulysin, were associated with the primary outcome and all-cause death in univariate analysis (all p < 0.01) and remained significantly associated in multivariable analysis adjusting for conventional clinical variables (e.g. age, gender, BMI, NYHA class, creatinine, LVEF, etiology) and CRP. However, after final adjustment for TnT and NT-proBNP no associations were found with outcomes. No interaction with darbepoetin alpha treatment was observed for any marker.
Conclusions
Leukocyte activation markers sCD163, MIF, sIL-2R, and ALCAM were associated with adverse outcome in patients with HFrEF, but add little as prognostic markers on top of established biochemical risk markers.
Clinical Trial Registration


Similar content being viewed by others
References
Frangogiannis NG (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11(5):255–265. https://doi.org/10.1038/nrcardio.2014.28
Vaduganathan M, Greene SJ, Butler J, Sabbah HN, Shantsila E, Lip GY, Gheorghiade M (2013) The immunological axis in heart failure: importance of the leukocyte differential. Heart Fail Rev 18(6):835–845. https://doi.org/10.1007/s10741-012-9352-9
Wrigley BJ, Lip GY, Shantsila E (2011) The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 13(11):1161–1171. https://doi.org/10.1093/eurjhf/hfr122
Radenovic S, Loncar G, Busjahn A, Apostolovic S, Zdravkovic M, Karlicic V, Veskovic J, Tahirovic E, Butler J, Dungen HD (2018) Systemic inflammation and functional capacity in elderly heart failure patients. Clin Res Cardiol 107(4):362–367. https://doi.org/10.1007/s00392-017-1195-x
Hofmann U, Frantz S (2015) Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 116(2):354–367. https://doi.org/10.1161/circresaha.116.304072
Swirski FK, Nahrendorf M (2013) Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339(6116):161–166. https://doi.org/10.1126/science.1230719
Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET (2014) Inflammatory cytokines as biomarkers in heart failure. Clin Chimica Acta Int J Clin Chem. https://doi.org/10.1016/j.cca.2014.09.001
Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AH, Goldberg LR, Jessup M, Cappola TP (2012) Multiple biomarkers for risk prediction in chronic heart failure. Circulation Heart Fail 5(2):183–190. https://doi.org/10.1161/CIRCHEARTFAILURE.111.965020
Chen YS, Gi WT, Liao TY, Lee MT, Lee SH, Hsu WT, Chang SS, Lee CC (2016) Using the galectin-3 test to predict mortality in heart failure patients: a systematic review and meta-analysis. Biomarkers Med 10(3):329–342. https://doi.org/10.2217/bmm.15.121
Gil VM, Ferreira JS (2014) Anemia and iron deficiency in heart failure. Rev Port Cardiol 33(1):39–44. https://doi.org/10.1016/j.repc.2013.06.003
von Haehling S, Gremmler U, Krumm M, Mibach F, Schon N, Taggeselle J, Dahm JB, Angermann CE (2017) Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP registry. Clin Res Cardiol 106(6):436–443. https://doi.org/10.1007/s00392-016-1073-y
Riedel O, Ohlmeier C, Enders D, Elsasser A, Vizcaya D, Michel A, Eberhard S, Schlothauer N, Berg J, Garbe E (2018) The contribution of comorbidities to mortality in hospitalized patients with heart failure. Clin Res Cardiol 107(6):487–497. https://doi.org/10.1007/s00392-018-1210-x
Nairz M, Theurl I, Wolf D, Weiss G (2016) Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia of inflammation. Wiener Medizinische Wochenschrift 166(13–14):411–423. https://doi.org/10.1007/s10354-016-0505-7
Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM (2002) Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol 72(4):711–717
Calandra T, Bernhagen J, Mitchell RA, Bucala R (1994) The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 179(6):1895–1902
Rubin LA, Kurman CC, Fritz ME, Biddison WE, Boutin B, Yarchoan R, Nelson DL (1985) Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol 135(5):3172–3177
Bowen MA, Patel DD, Li X, Modrell B, Malacko AR, Wang WC, Marquardt H, Neubauer M, Pesando JM, Francke U et al (1995) Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med 181(6):2213–2220
Tewary P, Yang D, de la Rosa G, Li Y, Finn MW, Krensky AM, Clayberger C, Oppenheim JJ (2010) Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood 116(18):3465–3474. https://doi.org/10.1182/blood-2010-03-273953
Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ, Committees R-H, Investigators R-H (2013) Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 368(13):1210–1219. https://doi.org/10.1056/NEJMoa1214865
McMurray JJ, Anand IS, Diaz R, Maggioni AP, O’Connor C, Pfeffer MA, Polu KR, Solomon SD, Sun Y, Swedberg K, Tendera M, van Veldhuisen DJ, Wasserman SM, Young JB (2009) Design of the reduction of events with darbepoetin alfa in heart failure (RED-HF): a phase III, anaemia correction, morbidity-mortality trial. Eur J Heart Fail 11(8):795–801. https://doi.org/10.1093/eurjhf/hfp098
McMurray JJ, Anand IS, Diaz R, Maggioni AP, O’Connor C, Pfeffer MA, Solomon SD, Tendera M, van Veldhuisen DJ, Albizem M, Cheng S, Scarlata D, Swedberg K, Young JB, Investigators R-HC (2013) Baseline characteristics of patients in the reduction of events with darbepoetin alfa in heart failure trial (RED-HF). Eur J Heart Fail 15(3):334–341. https://doi.org/10.1093/eurjhf/hfs204
Ptaszynska-Kopczynska K, Marcinkiewicz-Siemion M, Lisowska A, Waszkiewicz E, Witkowski M, Jasiewicz M, Miklasz P, Jakim P, Galar B, Musial WJ, Kaminski KA (2016) Alterations of soluble TWEAK and CD163 concentrations in patients with chronic heart failure. Cytokine 80:7–12. https://doi.org/10.1016/j.cyto.2016.02.005
Gullestad L, Ueland T, Brunsvig A, Kjekshus J, Simonsen S, Froland SS, Aukrust P (2001) Effect of metoprolol on cytokine levels in chronic heart failure—a substudy in the metoprolol controlled-release randomised intervention trial in heart failure (MERIT-HF). Am Heart J 141(3):418–421. https://doi.org/10.1067/mhj.2001.112785
Limas CJ, Hasikidis C, Iakovou J, Kroupis C, Haidaroglou A, Cokkinos DV (2003) Prognostic significance of soluble interleukin-2 receptor levels in patients with dilated cardiomyopathy. Eur J Clin Invest 33(6):443–448
Suthahar N, Meijers WC, Brouwers FP, Heerspink HJL, Gansevoort RT, van der Harst P, Bakker SJL, de Boer RA (2018) Heart failure and inflammation-related biomarkers as predictors of new-onset diabetes in the general population. Int J Cardiol 250:188–194. https://doi.org/10.1016/j.ijcard.2017.10.035
Anand IS (2008) Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol 52(7):501–511. https://doi.org/10.1016/j.jacc.2008.04.044
O’Meara E, Murphy C, McMurray JJ (2004) Anemia and heart failure. Curr Heart Fail Rep 1(4):176–182
Kristal B, Shurtz-Swirski R, Shasha SM, Manaster J, Shapiro G, Furmanov M, Hassan K, Weissman I, Sela S (1999) Interaction between erythropoietin and peripheral polymorphonuclear leukocytes in hemodialysis patients. Nephron 81(4):406–413. https://doi.org/10.1159/000045324
Sela S, Shurtz-Swirski R, Sharon R, Manaster J, Chezar J, Shkolnik G, Shapiro G, Shasha SM, Merchav S, Kristal B (2001) The polymorphonuclear leukocyte—a new target for erythropoietin. Nephron 88(3):205–210. https://doi.org/10.1159/000045991
Pesce M, Felaco P, Franceschelli S, Speranza L, Grilli A, De Lutiis MA, Ferrone A, Sirolli V, Bonomini M, Felaco M, Patruno A (2014) Effect of erythropoietin on primed leucocyte expression profile. Open Biol 4(6):140026. https://doi.org/10.1098/rsob.140026
Martiney JA, Sherry B, Metz CN, Espinoza M, Ferrer AS, Calandra T, Broxmeyer HE, Bucala R (2000) Macrophage migration inhibitory factor release by macrophages after ingestion of Plasmodium chabaudi-infected erythrocytes: possible role in the pathogenesis of malarial anemia. Infect Immun 68(4):2259–2267
McDevitt MA, Xie J, Ganapathy-Kanniappan S, Griffith J, Liu A, McDonald C, Thuma P, Gordeuk VR, Metz CN, Mitchell R, Keefer J, David J, Leng L, Bucala R (2006) A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J Exp Med 203(5):1185–1196. https://doi.org/10.1084/jem.20052398
Pereira R, Costa E, Goncalves M, Miranda V, do Sameiro Faria M, Quintanilha A, Belo L, Lima M, Santos-Silva A (2010) Neutrophil and monocyte activation in chronic kidney disease patients under hemodialysis and its relationship with resistance to recombinant human erythropoietin and to the hemodialysis procedure. Hemodial Int Symp Home Hemodial 14(3):295–301. https://doi.org/10.1111/j.1542-4758.2010.00450.x
Cooper AC, Breen CP, Vyas B, Ochola J, Kemeny DM, Macdougall IC (2003) Poor response to recombinant erythropoietin is associated with loss of T-lymphocyte CD28 expression and altered interleukin-10 production. Nephrol Dialysis Transplant 18(1):133–140
Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, Braunwald E (2007) Long-term prognostic value of neopterin: a novel marker of monocyte activation in patients with acute coronary syndrome. Circulation 115(24):3071–3078. https://doi.org/10.1161/CIRCULATIONAHA.106.666511
Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J, Walston J, Lange LA, Cushman M, Tracy RP (2013) Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arteriosclerosis Thromb Vascular Biol 33 (1):158–164. https://doi.org/10.1161/ATVBAHA.112.300421
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Inder Anand, John J. V. Mcmurray, Dirk J. van Veldhuisen and James Young are members of the RED-HF Executive Committee—no payments in the last 12 months. John J. V. Mcmurray has received travel and accommodation costs paid by Cytokinetics/Amgen in relation to advisory board and clinical trial meetings about omecamtiv mecarbil.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abraityte, A., Aukrust, P., Kou, L. et al. T cell and monocyte/macrophage activation markers associate with adverse outcome, but give limited prognostic value in anemic patients with heart failure: results from RED-HF. Clin Res Cardiol 108, 133–141 (2019). https://doi.org/10.1007/s00392-018-1331-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1331-2