Abstract
Purpose
The therapeutic effect of top-down therapy for inflammatory bowel disease (IBD) has not been fully evaluated in real-world clinical settings. We compared the effectiveness of top-down and step-up therapies for IBD.
Methods
We retrospectively evaluated patients who were admitted with IBD (Crohn’s disease [CD] or ulcerative colitis [UC]) between 2012 and 2019 using the nationwide Japan Diagnosis Procedure Combination database. Patients who received immunomodulators or biologic agents at the start of observation were assigned to the top-down group and those who did not were enrolled in the step-up group. Relapse was the primary outcome, a composite outcome defined as surgery, new steroid or immunomodulator use, hospitalization, a new biologic agent, or switching biologic agents.
Results
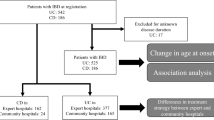
We analyzed 6715 patients (CD, N = 3643; UC, N = 3072). Relapse occurred in 1982 CD cases (54.4%).
The cumulative CD relapse incidence was 32.9% at 1 year and 61.3% at 5 years in the top-down group and 30.7% at 1 year and 58.6% at 5 years in the step-up group. Relapse occurred in 2032 UC cases (47.8%). The cumulative relapse incidence was 33.5% at 1 year and 50.0% at 5 years in the top-down group and 35.2% at 1 year and 51.6% at 5 years in the step-up group. No clinical factors associated with relapse were identified in patients with CD or UC.
Conclusion
Compared with step-up therapy, top-down therapy was not associated with a decreased relapse risk in a real-world population of patients with CD or UC.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140:1785–1794. https://doi.org/10.1053/j.gastro.2011.01.055
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF (2017) Ulcerative colitis. Lancet 389:1756–1770. https://doi.org/10.1016/S0140-6736(16)32126-2
Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L (2017) Crohn’s disease. Lancet 389:1741–1755. https://doi.org/10.1016/S0140-6736(16)31711-1
Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE (2018) ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 113:481–517. https://doi.org/10.1038/ajg.2018.27
Ruemmele FM, Veres G, Kolho KL et al (2014) Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 8:1179–1207. https://doi.org/10.1016/j.crohns.2014.04.005
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD (2019) ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 114:384–413. https://doi.org/10.14309/ajg.0000000000000152
Turner D, Levine A, Escher JC et al (2012) Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr 55:340–361. https://doi.org/10.1097/MPG.0b013e3182662233
Ungaro RC, Aggarwal S, Topaloglu O, Lee WJ, Clark R, Colombel JF (2020) Systematic review and meta-analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment Pharmacol Ther 51:831–842. https://doi.org/10.1111/apt.15685
D’Haens G, Baert F, van Assche G et al (2008) Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 371:660–667. https://doi.org/10.1016/S0140-6736(08)60304-9
Khanna R, Bressler B, Levesque BG et al (2015) Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 386:1825–1834. https://doi.org/10.1016/S0140-6736(15)00068-9
Szklo M (1998) Population-based cohort studies. Epidemiol Rev 20:81–90. https://doi.org/10.1093/oxfordjournals.epirev.a017974
Booth CM, Tannock IF (2014) Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer 110:551–555. https://doi.org/10.1038/bjc.2013.725
Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ (2010) The natural history of adult Crohn’s disease in population-based cohorts. Am J Gasroenterol 105:289–297. https://doi.org/10.1038/ajg.2009.579
Kennedy NA, Kalla R, Warner B et al (2014) Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther 40:1313–1323. https://doi.org/10.1111/apt.12980
Dulai PS, Singh S, Vande Casteele N et al (2019) Should we divide Crohn’s disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol 17:2634–2643. https://doi.org/10.1016/j.cgh.2019.04.040
Burisch J, Kiudelis G, Kupcinskas L et al (2019) Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut 68:423–433. https://doi.org/10.1136/gutjnl-2017-315568
Magro F, Rodrigues-Pinto E, Coelho R et al (2014) Is it possible to change phenotype progression in Crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol 109:1026–1036. https://doi.org/10.1038/ajg.2014.97
Iborra M, Pérez-Gisbert J, Bosca-Watts MM et al (2017) Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naïve and non-naïve patients. J Gastroenterol 52:788–799. https://doi.org/10.1007/s00535-016-1274-1
Vester-Andersen MK, Vind I, Prosberg MV et al (2014) Hospitalisation, surgical and medical recurrence rates in inflammatory bowel disease 2003–2011—a Danish population-based cohort study. J Crohns Colitis 8:1675–1683. https://doi.org/10.1016/j.crohns.2014.07.010
Jeuring SF, van den Heuvel TR, Liu LY et al (2017) Improvements in the long-term outcome of Crohn’s disease over the past two decades and the relation to changes in medical management: results from the population-based IBDSL cohort. Am J Gastroenterol 112:325–336. https://doi.org/10.1038/ajg.2016.524
Acknowledgements
Data for this study was provided by the Database Center of the National University Hospitals, University of Tokyo Hospital. Use of the data was approved by the Database Center Management Committee, National University Hospital Council of Japan (No. 2020-K001: 1/19/2021).
Author information
Authors and Affiliations
Contributions
Ochi M, Niikura R, Otsubo T, Yamada A, Kawai T, and Koike K contributed equally to this work. Ochi M, Niikura R, and Otsubo T collected and analyzed the data. Ochi M drafted the manuscript. Ochi M and Niikura R designed and supervised the study. The statistical methods of this study were reviewed by Otsubo T. Yamada A, Kawai T, and Koike K offered technical or material support. All authors have read and approved the final version to be published.
Corresponding authors
Ethics declarations
Ethics approval
The University of Tokyo Hospital Institutional Review Board approved the study (2058–2). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate and for publication
The University of Tokyo Hospital Institutional Review Board waived the requirement for patient informed consent because we used anonymized data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ochi, M., Niikura, R., Otsubo, T. et al. Comparison of inflammatory bowel disease relapse after top-down or step-up therapy: a population-based cohort study. Int J Colorectal Dis 36, 2227–2235 (2021). https://doi.org/10.1007/s00384-021-04007-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-04007-4