Abstract
Purpose
This study aimed to investigate in a multicenter cohort study the radicality of colorectal cancer resections, to assess the oncosurgical quality of colorectal specimens, and to compare the performance between centers.
Methods
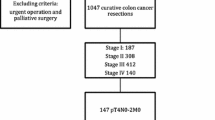
One German and nine Swiss hospitals agreed to prospectively register all patients with primary colorectal cancer resected between September 2001 and June 2005. The median number of eligible patients with one primary tumor included per center was 95 (range 12–204).
Results
The following variations of median values or percentages between centers were found: length of bowel specimen 20–39 cm (25.8 cm), maximum height of mesocolon 6.5–12.5 cm (9.0 cm), number of examined lymph nodes 9–24 (16), distance to nearer bowel resection margin in colon cancer 4.8–12 cm (7 cm), and in rectal cancer 2–3 cm (2.5 cm), central ligation of major artery 40–97 % (71 %), blood loss 200–500 ml (300 ml), need for perioperative blood transfusion 5–40 % (19 %), tumor opened during mobilization 0–11 % (5 %), T4-tumors not en-bloc resected 0–33 % (4 %), inadvertent perforation of mesocolon/mesorectum 0–8 % (4 %), no-touch isolation technique 36–86 % (67 %), abdominoperineal resection for rectal cancer 0–30 % (17 %), rectal cancer specimen with circumferential margin ≤1 mm 0–19 % (10 %), in-hospital mortality 0–6 % (2 %), anastomotic leak or intra-abdominal abscess 0–17 % (7 %), re-operation 0–17 % (8 %).
Conclusion
In colorectal cancer, surgery considerable variations between different centers were found with regard to radicality and oncosurgical quality, suggesting a potential for targeted improvement of surgical technique.


Similar content being viewed by others
References
Renzulli P, Lowy A, Maibach R, Egeli RA, Metzger U, Laffer UT (2006) The influence of the surgeon’s and the hospital’s caseload on survival and local recurrence after colorectal cancer surgery. Surgery 139(3):296–304
Dorrance HR, Docherty GM, O’Dwyer PJ (2000) Effect of surgeon specialty interest on patient outcome after potentially curative colorectal cancer surgery. Dis Colon rectum 43(4):492–498
Iversen LH, Harling H, Laurberg S, Wille-Jorgensen P, Danish Colorectal Cancer G (2007) Influence of caseload and surgical speciality on outcome following surgery for colorectal cancer: a review of evidence. Part 2: long-term outcome. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 9(1):38–46
Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP (1984) Local recurrence following ‘curative’ surgery for large bowel cancer: I. The overall picture. The British journal of surgery 71(1):12–16
Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP (1984) Local recurrence following ‘curative’ surgery for large bowel cancer: II. The rectum and rectosigmoid. The British journal of surgery 71(1):17–20
McArdle CS, Hole D (1991) Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. BMJ 302(6791):1501–1505
Porter GA, Soskolne CL, Yakimets WW, Newman SC (1998) Surgeon-related factors and outcome in rectal cancer. Ann Surg 227(2):157–167
Borowski DW, Kelly SB, Bradburn DM, Wilson RG, Gunn A, Ratcliffe AA, Northern Region Colorectal Cancer Audit G (2007) Impact of surgeon volume and specialization on short-term outcomes in colorectal cancer surgery. The British journal of surgery 94(7):880–889
Iversen LH, Harling H, Laurberg S, Wille-Jorgensen P (2007) Influence of caseload and surgical speciality on outcome following surgery for colorectal cancer: a review of evidence. Part 1: short-term outcome. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 9(1):28–37
Association of Coloproctology of Great Britain and Ireland (2007) Guidelines for the management of colorectal cancer. 3rd edn.
Otchy D, Hyman NH, Simmang C, Anthony T, Buie WD, Cataldo P, Church J, Cohen J, Dentsman F, Ellis CN, Kilkenny JW III, Ko C, Moore R, Orsay C, Place R, Rafferty J, Rakinic J, Savoca P, Tjandra J, Whiteford M, Standards Practice Task F, American Society of C, Rectal S (2004) Practice parameters for colon cancer. Dis Colon rectum 47(8):1269–1284
Tjandra JJ, Kilkenny JW, Buie WD, Hyman N, Simmang C, Anthony T, Orsay C, Church J, Otchy D, Cohen J, Place R, Denstman F, Rakinic J, Moore R, Whiteford M, Standards Practice Task F, American Society of C, Rectal S (2005) Practice parameters for the management of rectal cancer (revised). Dis Colon rectum 48(3):411–423
Schmiegel W, Reinacher-Schick A, Arnold D, Graeven U, Heinemann V, Porschen R, Riemann J, Rodel C, Sauer R, Wieser M, Schmitt W, Schmoll HJ, Seufferlein T, Kopp I, Pox C (2008) Update S3-guideline “colorectal cancer” 2008. Zeitschrift fur Gastroenterologie 46(8):799–840
Maurer CA (2004) Colon cancer: resection standards. Techniques in coloproctology 8(Suppl 1):s29–s32
Maurer CA, Renzulli P, Meyer JD, Buchler MW (1999) Rectal carcinoma. Optimizing therapy by partial or total mesorectum removal. Zentralblatt fur Chirurgie 124(5):428–435
Sobin LHWC UICC International Union Against Cancer TNM classification of malignant tumours, 6th edn. Wiley, Lissabon, New York
Turnbull RB Jr, Kyle K, Watson FR, Spratt J (1967) Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg 166(3):420–427
Goldstein NS, Soman A, Sacksner J (1999) Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol 111(3):349–351
Kwok SP, Lau WY, Leung KL, Liew CT, Li AK (1996) Prospective analysis of the distal margin of clearance in anterior resection for rectal carcinoma. The British journal of surgery 83(7):969–972
Weese JL, O’Grady MG, Ottery FD (1986) How long is the five centimeter margin? Surgery, gynecology & obstetrics 163(2):101–103
Neufeld D, Bugyev N, Grankin M, Gutman M, Klein E, Bernheim J, Shpitz B (2007) Specimen length as a perioperative surrogate marker for adequate lymphadenectomy in colon cancer: the surgeon's role. Int Surg 92(3):155–160
Wang J, Kulaylat M, Rockette H, Hassett J, Rajput A, Dunn KB, Dayton M (2009) Should total number of lymph nodes be used as a quality of care measure for stage III colon cancer? Ann Surg 249(4):559–563
Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG (2003) Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 21(15):2912–2919
Chen SL, Bilchik AJ (2006) More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg 244(4):602–610
van Steenbergen LN, van Lijnschoten G, Rutten HJ, Lemmens VE, Coebergh JW (2010) Improving lymph node detection in colon cancer in community hospitals and their pathology department in southern Netherlands. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 36(2):135–140
Wright FC, Law CH, Last L, Khalifa M, Arnaout A, Naseer Z, Klar N, Gallinger S, Smith AJ (2003) Lymph node retrieval and assessment in stage II colorectal cancer: a population-based study. Ann Surg Oncol 10(8):903–909
Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, Grebner A, Ulm K, Hofler H, Nekarda H, Siewert JR (2008) Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg 248(6):968–978
Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG (2005) Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23(34):8706–8712
Peschaud F, Benoist S, Julie C, Beauchet A, Penna C, Rougier P, Nordlinger B (2008) The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg 248(6):1067–1073
Vaccaro CA, Im V, Rossi GL, Quintana GO, Benati ML, Perez de Arenaza D, Bonadeo FA (2009) Lymph node ratio as prognosis factor for colon cancer treated by colorectal surgeons. Dis Colon rectum 52(7):1244–1250
Hida J, Okuno K, Yasutomi M, Yoshifuji T, Uchida T, Tokoro T, Shiozaki H (2005) Optimal ligation level of the primary feeding artery and bowel resection margin in colon cancer surgery: the influence of the site of the primary feeding artery. Dis Colon rectum 48(12):2232–2237
West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P (2008) Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. The lancet oncology 9(9):857–865
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation-technical notes and outcome. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 11(4):354–364
Read TE, Mutch MG, Chang BW, McNevin MS, Fleshman JW, Birnbaum EH, Fry RD, Caushaj PF, Kodner IJ (2002) Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg 195(1):33–40
Slanetz CA Jr, Grimson R (1997) Effect of high and intermediate ligation on survival and recurrence rates following curative resection of colorectal cancer. Dis Colon rectum 40(10):1205–1218
Toyota S, Ohta H, Anazawa S (1995) Rationale for extent of lymph node dissection for right colon cancer. Dis Colon rectum 38(7):705–711
Morikawa E, Yasutomi M, Shindou K, Matsuda T, Mori N, Hida J, Kubo R, Kitaoka M, Nakamura M, Fujimoto K et al (1994) Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis Colon rectum 37(3):219–223
Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J (1993) Blood transfusions and prognosis in colorectal cancer. N Engl J Med 328(19):1372–1376
Morris E, Quirke P, Thomas JD, Fairley L, Cottier B, Forman D (2008) Unacceptable variation in abdominoperineal excision rates for rectal cancer: time to intervene? Gut 57(12):1690–1697
Zirngibl H, Husemann B, Hermanek P (1990) Intraoperative spillage of tumor cells in surgery for rectal cancer. Dis Colon rectum 33(7):610–614
Slanetz CA Jr (1984) The effect of inadvertent intraoperative perforation on survival and recurrence in colorectal cancer. Dis Colon rectum 27(12):792–797
Eriksen MT, Wibe A, Syse A, Haffner J, Wiig JN, Norwegian Rectal Cancer G, Norwegian Gastrointestinal Cancer G (2004) Inadvertent perforation during rectal cancer resection in Norway. The British journal of surgery 91(2):210–216
Maurer CA, Renzulli P, Kull C, Kaser SA, Mazzucchelli L, Ulrich A, Buchler MW (2011) The impact of the introduction of total mesorectal excision on local recurrence rate and survival in rectal cancer: long-term results. Ann Surg Oncol 18(7):1899–1906
Maurer CA (2005) Urinary and sexual function after total mesorectal excision. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 165:196–204
Maurer CA, Z'Graggen K, Renzulli P, Schilling MK, Netzer P, Buchler MW (2001) Total mesorectal excision preserves male genital function compared with conventional rectal cancer surgery. The British journal of surgery 88(11):1501–1505
Acknowledgments
We thank the co-workers of the pathology institutes of Liestal, Chur, Bern, Zürich, Luzern, all in Switzerland, and of Homburg-Saar, Germany, for their excellent collaboration in examination of all the colorectal specimens in a standardized manner and in completing the pathology forms. Furthermore, we thank Mr. Michael Mayer and Mrs. Hong Sun for the statistical support and Mrs. E. Hansen and L. Kacina for data collection, all from the SAKK Coordinating Center in Bern. The study was financially supported by the Swiss State Secretariat for Education and Research (SER).
Author information
Authors and Affiliations
Corresponding author
Additional information
Christian Klaiber is deceased October 2013
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Maurer, C.A., Dietrich, D., Schilling, M.K. et al. Prospective multicenter registration study of colorectal cancer: significant variations in radicality and oncosurgical quality—Swiss Group for Clinical Cancer Research Protocol SAKK 40/00. Int J Colorectal Dis 32, 57–74 (2017). https://doi.org/10.1007/s00384-016-2667-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2667-6