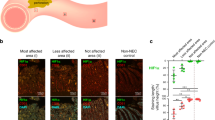
Abstract
Purpose
Remote ischemic conditioning (RIC) is a maneuver involving brief cycles of ischemia reperfusion in an individual’s limb. In the early stage of experimental NEC, RIC decreased intestinal injury and prolonged survival by counteracting the derangements in intestinal microcirculation. A single-center phase I study demonstrated that the performance of RIC was safe in neonates with NEC. The aim of this phase II RCT was to evaluate the safety and feasibility of RIC, to identify challenges in recruitment, retainment, and to inform a phase III RCT to evaluate efficacy.
Methods
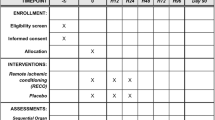
RIC will be performed by trained research personnel and will consist of four cycles of limb ischemia (4-min via cuff inflation) followed by reperfusion (4-min via cuff deflation), repeated on two consecutive days post randomization. The primary endpoint of this RCT is feasibility and acceptability of recruiting and randomizing neonates within 24 h from NEC diagnosis as well as masking and completing the RIC intervention.
Results
We created a novel international consortium for this trial and created a consensus on the diagnostic criteria for NEC and protocol for the trial. The phase II multicenter-masked feasibility RCT will be conducted at 12 centers in Canada, USA, Sweden, The Netherlands, UK, and Spain. The inclusion criteria are: gestational age < 33 weeks, weight ≥ 750 g, NEC receiving medical treatment, and diagnosis established within previous 24 h. Neonates will be randomized to RIC (intervention) or no-RIC (control) and will continue to receive standard management of NEC. We expect to recruit and randomize 40% of eligible patients in the collaborating centers (78 patients; 39/arm) in 30 months. Bayesian methods will be used to combine uninformative prior distributions with the corresponding observed proportions from this trial to determine posterior distributions for parameters of feasibility.
Conclusions
The newly established NEC consortium has generated novel data on NEC diagnosis and defined the feasibility parameters for the introduction of a novel treatment in NEC. This phase II RCT will inform a future phase III RCT to evaluate the efficacy and safety of RIC in early-stage NEC.

Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Alganabi, M., Lee, C., Bindi, E., Li, B. & Pierro, A. Recent advances in understanding necrotizing enterocolitis. F1000Res 8, https://doi.org/10.12688/f1000research.17228.1 (2019).
Neu J, Walker WA (2011) Necrotizing enterocolitis. N Engl J Med 364:255–264. https://doi.org/10.1056/NEJMra1005408
Rees CM, Pierro A, Eaton S (2007) Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed 92:F193-198. https://doi.org/10.1136/adc.2006.099929
Nino, D. F. et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci Transl Med 10, https://doi.org/10.1126/scitranslmed.aan0237 (2018).
Squires, R. H. et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 161, 723–728 e722, https://doi.org/10.1016/j.jpeds.2012.03.062 (2012).
Robinson JR et al (2017) Surgical necrotizing enterocolitis. Semin Perinatol 41:70–79. https://doi.org/10.1053/j.semperi.2016.09.020
Jones, I. H. & Hall, N. J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis-A Systematic Review. J Pediatr 220, 86–92 e83, https://doi.org/10.1016/j.jpeds.2019.11.011 (2020).
Chen Y et al (2016) Formula feeding and systemic hypoxia synergistically induce intestinal hypoxia in experimental necrotizing enterocolitis. Pediatr Surg Int 32:1115–1119. https://doi.org/10.1007/s00383-016-3997-8
Hsueh W et al (2003) Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 6:6–23. https://doi.org/10.1007/s10024-002-0602-z
Chen, Y. et al. Formula feeding and immature gut microcirculation promote intestinal hypoxia, leading to necrotizing enterocolitis. Dis Model Mech 12, https://doi.org/10.1242/dmm.040998 (2019).
Kosloske A (1994) Epidemiology of necrotizing enterocolitis. Acta Paediatr 83:2–7. https://doi.org/10.1111/j.1651-2227.1994.tb13232.x
Llanos AR et al (2002) Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol 16:342–349. https://doi.org/10.1046/j.1365-3016.2002.00445.x
Guthrie SO et al (2003) Necrotizing enterocolitis among neonates in the United States. J Perinatol 23:278–285. https://doi.org/10.1038/sj.jp.7210892
Stout G et al (2008) Necrotizing enterocolitis during the first week of life: a multicentered case-control and cohort comparison study. J Perinatol 28:556–560. https://doi.org/10.1038/jp.2008.36
Koike Y et al (2020) Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat Commun 11:4950. https://doi.org/10.1038/s41467-020-18750-9
Havranek T, Rahimi M, Hall H, Armbrecht E (2015) Feeding preterm neonates with patent ductus arteriosus (PDA): intestinal blood flow characteristics and clinical outcomes. J Maternal-Fetal Neonatal Med 28:526–530. https://doi.org/10.3109/14767058.2014.923395
Sieber C et al (1991) Regulation of postprandial mesenteric blood flow in humans: evidence for a cholinergic nervous reflex. Gut 32:361–366. https://doi.org/10.1136/gut.32.4.361
Fang S, Kempley ST, Gamsu HR (2001) Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed 85:F42-45. https://doi.org/10.1136/fn.85.1.f42
Downard CD et al (2011) Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. J Pediatr Surg 46:1023–1028. https://doi.org/10.1016/j.jpedsurg.2011.03.023
Yazji I et al (2013) Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci USA 110:9451–9456. https://doi.org/10.1073/pnas.1219997110
Yu X, Radulescu A, Zorko N, Besner GE (2009) Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology 137:221–230. https://doi.org/10.1053/j.gastro.2009.03.060
Good M et al (2016) The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr 116:1175–1187. https://doi.org/10.1017/S0007114516002944
Zani A et al (2016) A spectrum of intestinal injury models in neonatal mice. Pediatr Surg Int 32:65–70. https://doi.org/10.1007/s00383-015-3813-x
Eitel I et al (2015) Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 36:3049–3057. https://doi.org/10.1093/eurheartj/ehv463
Botker HE et al (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. https://doi.org/10.1016/S0140-6736(09)62001-8
Gaspar A et al (2018) Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol 113:14. https://doi.org/10.1007/s00395-018-0672-3
Kang Z et al (2018) Remote ischemic preconditioning upregulates microRNA-21 to protect the kidney in children with congenital heart disease undergoing cardiopulmonary bypass. Pediatr Nephrol 33:911–919. https://doi.org/10.1007/s00467-017-3851-9
Zhong H et al (2013) Cardioprotective effect of remote ischemic postconditioning on children undergoing cardiac surgery: a randomized controlled trial. Paediatr Anaesth 23:726–733. https://doi.org/10.1111/pan.12181
Luo W, Zhu M, Huang R, Zhang Y (2011) A comparison of cardiac post-conditioning and remote pre-conditioning in paediatric cardiac surgery. Cardiol Young 21:266–270. https://doi.org/10.1017/S1047951110001915
Zhou W et al (2010) Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol 31:22–29. https://doi.org/10.1007/s00246-009-9536-9
Cheung MM et al (2006) Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47:2277–2282. https://doi.org/10.1016/j.jacc.2006.01.066
Zhao JJ et al (2018) Remote Ischemic Postconditioning for Ischemic Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Chin Med J 131:956–965. https://doi.org/10.4103/0366-6999.229892
Hausenloy DJ et al (2019) Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet 394:1415–1424. https://doi.org/10.1016/S0140-6736(19)32039-2
Meybohm P et al (2015) A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 373:1397–1407. https://doi.org/10.1056/NEJMoa1413579
Hausenloy DJ et al (2015) Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 373:1408–1417. https://doi.org/10.1056/NEJMoa1413534
Li C et al (2013) Limb remote ischemic preconditioning for intestinal and pulmonary protection during elective open infrarenal abdominal aortic aneurysm repair: a randomized controlled trial. Anesthesiology 118:842–852. https://doi.org/10.1097/ALN.0b013e3182850da5
Struck R et al (2018) Effect of remote ischemic preconditioning on intestinal ischemia-reperfusion injury in adults undergoing on-pump CABG surgery: a randomized controlled pilot trial. J Cardiothorac Vasc Anesth 32:1243–1247. https://doi.org/10.1053/j.jvca.2017.07.027
Godskesen LE et al (2020) Remote ischemic conditioning in active ulcerative colitis: an explorative randomized clinical trial. Sci Rep 10:9537. https://doi.org/10.1038/s41598-020-65692-9
Zozaya C et al. Remote ischemic conditioning in preterm neonates with necrotizing enterocolitis: a phase i feasibility and safety nonrandomized clinical trial. Submitted for publication.
Ji J et al (2014) A data-driven algorithm integrating clinical and laboratory features for the diagnosis and prognosis of necrotizing enterocolitis. PLoS ONE 9:e89860. https://doi.org/10.1371/journal.pone.0089860
Fogel DB (2018) Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp Clin Trials Commun 11:156–164. https://doi.org/10.1016/j.conctc.2018.08.001
Moss RL et al (2006) Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med 354:2225–2234. https://doi.org/10.1056/NEJMoa054605
Rees CM et al (2008) Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg 248:44–51. https://doi.org/10.1097/SLA.0b013e318176bf81
Chan A-W et al (2013) SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Ann Intern Med 158(3):200–207. https://doi.org/10.7326/0003-4819-158-3-201302050-00583
Carnaghan H et al (2020) Antenatal corticosteroids and outcomes in gastroschisis: a multicenter retrospective cohort study. Prenat Diagn 40:991–997. https://doi.org/10.1002/pd.5727
Hall NJ et al (2015) Outcome reporting in randomised controlled trials and meta-analyses of appendicitis treatments in children: a systematic review. Trials 16:275. https://doi.org/10.1186/s13063-015-0783-1
Faingold R et al (2005) Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology 235:587–594. https://doi.org/10.1148/radiol.2352031718
Svensson JF et al (2015) Nonoperative treatment with antibiotics versus surgery for acute nonperforated appendicitis in children: a pilot randomized controlled trial. Ann Surg 261:67–71. https://doi.org/10.1097/SLA.0000000000000835
Svensson, J. F. et al. Design of Studies for Antibiotic Treatment of Acute Appendicitis in Children: In Support of RCTs. Annals of surgery 266, e6-e7, https://doi.org/10.1097/SLA.0000000000001291 (2017).
Hall NJ et al. Appendectomy versus non-operative treatment for acute uncomplicated appendicitis in children: study protocol for a multicentre, open-label, non-inferiority, randomised controlled trial. BMJ Paediatr Open 1, https://doi.org/10.1136/bmjpo-2017-000028 (2017).
Zani A et al (2015) International survey on the management of necrotizing enterocolitis. Eur J Pediatr Surg 25:27–33. https://doi.org/10.1055/s-0034-1387942
Bishay M et al (2020) The effect of glutamine supplementation on microbial invasion in surgical infants requiring parenteral nutrition: results of a randomized controlled trial. JPEN J Parenter Enteral Nutr 44:80–91. https://doi.org/10.1002/jpen.1700
Singh RR et al (2017) Double-blind randomized clinical trial of percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy in children. Br J Surg 104:1620–1627. https://doi.org/10.1002/bjs.10687
Hall NJ et al (2017) Active observation versus interval appendicectomy after successful non-operative treatment of an appendix mass in children (CHINA study): an open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2:253–260. https://doi.org/10.1016/S2468-1253(16)30243-6
Pacilli M et al (2014) Four year follow-up of a randomised controlled trial comparing open and laparoscopic Nissen fundoplication in children. Arch Dis Child 99:516–521. https://doi.org/10.1136/archdischild-2013-304279
Bishay M et al (2013) Hypercapnia and acidosis during open and thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia: results of a pilot randomized controlled trial. Ann Surg 258:895–900. https://doi.org/10.1097/SLA.0b013e31828fab55
Eaton S, Klein N, Ong E, Pierro A (2013) Authors’ reply: Randomized clinical trial of glutamine-supplemented versus standard parenteral nutrition in infants with surgical gastrointestinal disease (Br J Surg 2012; 99: 929–938). Br J Surg 100:841–842. https://doi.org/10.1002/bjs.9126
Ong EG et al (2012) Randomized clinical trial of glutamine-supplemented versus standard parenteral nutrition in infants with surgical gastrointestinal disease. Br J Surg 99:929–938. https://doi.org/10.1002/bjs.8750
McHoney M et al (2011) Clinical outcome of a randomized controlled blinded trial of open versus laparoscopic Nissen fundoplication in infants and children. Ann Surg 254:209–216. https://doi.org/10.1097/SLA.0b013e318226727f
McHoney M. et al. Effect of laparoscopy and laparotomy on energy and protein metabolism in children: a randomized controlled trial. J Pediatr 157, 439–444, 444 e432, https://doi.org/10.1016/j.jpeds.2010.02.067 (2010).
Rees, C. M. et al. Peritoneal drainage does not stabilize extremely low birth weight infants with perforated bowel: data from the NET Trial. J Pediatr Surg 45, 324–328; discussion 328–329, https://doi.org/10.1016/j.jpedsurg.2009.10.066 (2010).
McHoney, M. et al. Inflammatory response in children after laparoscopic vs open Nissen fundoplication: randomized controlled trial. J Pediatr Surg 40, 908–913; discussion 913–904, https://doi.org/10.1016/j.jpedsurg.2005.03.003 (2005).
Hall NJ et al (2009) Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet (London, England) 373:390–398. https://doi.org/10.1016/s0140-6736(09)60006-4
Canadian Neonatal Network. Canadian Neonatal Network Report. (2019).
Battersby C, Longford N, Costeloe K, Modi N, Group UKN. C. N. E. S. Development of a Gestational Age-Specific Case Definition for Neonatal Necrotizing Enterocolitis. JAMA Pediatr 171, 256–263, doi:https://doi.org/10.1001/jamapediatrics.2016.3633 (2017).
McNair C et al (2004) Postoperative pain assessment in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed 89:F537-541. https://doi.org/10.1136/adc.2003.032961
Stevens B et al (1996) Premature Infant Pain Profile: development and initial validation. Clin J Pain 12:13–22. https://doi.org/10.1097/00002508-199603000-00004
Wynn JL et al (2021) Multicenter validation of the neonatal sequential organ failure assessment score for prognosis in the neonatal intensive care unit. J Pediatr. https://doi.org/10.1016/j.jpeds.2021.05.037
Fleiss N et al (2021) Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw Open 4:e2036518. https://doi.org/10.1001/jamanetworkopen.2020.36518
Fisher JG et al (2014) Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J Pediatr Surg 49:1215–1219. https://doi.org/10.1016/j.jpedsurg.2013.11.051
Kirtsman M et al (2015) Nil-per-os days and necrotizing enterocolitis in extremely preterm infants. Am J Perinatol 32:785–794. https://doi.org/10.1055/s-0034-1396687
Rees CM et al (2005) Surgical strategies for necrotising enterocolitis: a survey of practice in the United Kingdom. Arch Dis Child Fetal Neonatal Ed 90:F152-155. https://doi.org/10.1136/adc.2004.051862
Guillot M, Chau V, Lemyre B (2020) Routine imaging of the preterm neonatal brain. Paediatr Child Health 25:249–262. https://doi.org/10.1093/pch/pxaa033
Chiang MF et al (2021) International classification of retinopathy of prematurity. Third Edition Ophthalmology 128(10):e51–e68. https://doi.org/10.1016/j.ophtha.2021.05.031
Willan AR, Thabane L (2020) Bayesian methods for pilot studies. Clin Trials 17:414–419. https://doi.org/10.1177/1740774520914306
A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 365, 711-722, doi:https://doi.org/10.1016/s0140-6736(05)17965-3 (2005).
Hartling L et al (2011) StaR Child Health: developing evidence-based guidance for the design, conduct, and reporting of pediatric trials. Clin Pharmacol Ther 90:727–731. https://doi.org/10.1038/clpt.2011.212
Ellenberg S et al (2012) Standard 3: data monitoring committees. Pediatrics 129(Suppl 3):S132-137. https://doi.org/10.1542/peds.2012-0055F
Schandelmaier S et al. Premature discontinuation of pediatric randomized controlled trials: a retrospective cohort study. J Pediatr 184: 209–214 e201, doi:https://doi.org/10.1016/j.jpeds.2017.01.071 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest has been declared by the authors in relation to the study itself.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ganji, N., Li, B., Ahmad, I. et al. Remote ischemic conditioning in necrotizing enterocolitis: study protocol of a multi-center phase II feasibility randomized controlled trial. Pediatr Surg Int 38, 679–694 (2022). https://doi.org/10.1007/s00383-022-05095-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05095-1