Abstract
Introduction
The growth factor, ‘Glial cell line-Derived Neurotrophic Factor’ (GDNF), is involved in the development of enteric ganglia, using the tyrosine kinase receptor ‘REarranged during Transfection’ (RET) to stimulate the proliferation and differentiation of neural crest-derived precursor cells. To date, the presence of these signalling molecules have not been studied in the developing cloaca, thus the aim of this study was to investigate the distribution of RET and GDNF, and analyse their co-localisation in vagal-derived neurons of the cloaca using quail-chick chimera embryos.
Materials and methods
Chicken embryos were incubated until the 10–12 somite stage. The vagal neural tube was microsurgically ablated in ovo and replaced with the vagal neural tube from age-matched quail embryos. Quail-chick chimera embryos were harvested at E12, fixed and embedded in paraffin wax, and serially sectioned. Immunohistochemistry was performed using human natural killer-1 (HNK-1), quail-cell-specific perinuclear (QCPN), GDNF and RET antibodies.
Results
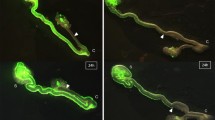
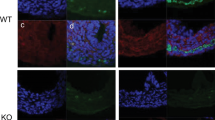
HNK-1 labelled all ganglia in the myenteric and submucosal plexuses of the cloaca, while the quail-specific QCPN antibody labelled all ganglia derived from the transplanted quail vagal neural tube (Fig. 1, a, b). RET and GDNF were found both co-localised and expressed in separate ganglia in the cloaca (Fig. 1, c, d). The majority of QCPN-labelled vagal-derived neurons also expressed RET and GDNF.

HNK-1 (a), QCPN (b), GDNF (c) and RET (d) immunoreactivity in the chick cloaca at E12. Arrows show ganglia displaying co-immunoreactivity for all four antibodies
Conclusion
Results show that GDNF and RET signalling play a major role in ENS development in the chick embryo cloaca. We have shown, for the first time, that the majority of vagal neural crest-derived neurons co-express RET and GDNF, thus highlighting the importance of these signalling factors in cloacal development.
Similar content being viewed by others
References
Montgomery RK, Mulberg AE, Grand RJ (1999) Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 116:702–731
Schuchardt A, D’Agati V, Larsson-Blomberg L et al (1994) Defects in the kidney and enteric nervous system in mice lacking the tyrosine kinase receptor Ret. Nature 367:380–383
Kusafuka T, Puri P (1997) The RET proto-oncogene: a challenge to our understanding of disease pathogenesis. Pediatr Surg Int 12:11–18
Martucciello G, Ceccherini I, Lerone M et al (2000) Pathogenesis of Hirschsprung’s disease. J Pediatr Surg 35:1017–1025
Hellmich HL, Kos L, Cho ES et al (1996) Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev 54:95–105
Worley D, Pisano J, Choi E et al (2000) Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development 127:4383–4393
Young H, Hearn C, Farlie P et al (2001) GDNF is a chemoattractant for enteric neural cells. Dev Biol 229:503–516
Sanchez MP, Silos-Santiago I, Frisen J et al (1996) Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382:70–73
Pichel J, Shen L, Sheng H et al (1996) Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382:73–76
Mo R, Kim JH, Zhang J et al (2001) Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Path 159:765–774
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–67
O’Donnell AM, Bannigan J, Puri P (2005) The effect of vagal neural crest ablation on the chick embryo cloaca. Pediatr Surg Int 21:180–183
Guesdon JL, Ternynck T, Avrameas S (1979) The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem 27:1131–1139
Le Douarin NM, Teillet MA (1973) The migration of neural crest cells to the walls of the digestive tract in the avian embryo. J Embryol Exp Biol 30:31–48
Le Douarin NM, Teillet MA (1974) Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and neuroectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol 41:162–184
Pomeranz HD, Gershon MD (1990) Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev Biol 137:378–394
Pomeranz HD, Rothman TP, Gershon MD (1991) Colonization of the post-umbilical bowel by cells derived from the sacral neural crest: direct tracing of cell migration using an intercalated probe and a replication-deficient retrovirus. Development 111:647–655
O’ Donnell A, Bannigan J, Puri P (2006) Vagal neural crest contribution to the chick embryo cloaca. Pediatr Surg Int 22:983–986
Taraviras S, Marcos-Gutierrez C, Durbec P et al (1999) Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 126:2785–2797
Schiltz C, Benjamin J, Epstein M (1999) Expression of the GDNF receptors ret and GFR-alpha1 in the developing avian enteric nervous system. J Comp Neurol 414:193–211
Delalande JM, Barlow A, Thomas A et al (2008) The receptor tyrosine kinase RET regualtes hindgut colonization by sacral neural crest cells. Dev Biol 313:279–292
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’ Donnell, AM., Puri, P. RET/GDNF signalling in vagal neural crest-derived neurons of the chick embryo cloaca. Pediatr Surg Int 24, 1211–1214 (2008). https://doi.org/10.1007/s00383-008-2237-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-008-2237-2