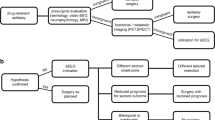
Abstract
Introduction
Stereoelectroencephalography (SEEG) is valuable for delineating the seizure onset zone (SOZ) in pharmacoresistant epilepsy when non-invasive presurgical techniques are inconclusive. Secondary epilepsy surgery after initial failure is challenging and there is limited research on SEEG following failed epilepsy surgery in children.
Objective
The objective of this manuscript is to present the outcomes of children who underwent SEEG after failed epilepsy surgery.
Methods
In this single-institution retrospective study, demographics, previous surgery data, SEEG characteristics, management, and follow-up were analyzed for pediatric patients who underwent SEEG after unsuccessful epilepsy surgery between August 2016 and February 2023.
Results
Fifty three patients underwent SEEG investigation during this period. Of this, 13 patients were identified who had unsuccessful initial epilepsy surgery (24%). Of these 13 patients, six patients (46%) experienced unsuccessful resective epilepsy surgery that targeted the temporal lobe, six patients (46%) underwent surgery involving the frontal lobe, and one patient (8%) had laser interstitial thermal therapy (LITT) of the right insula. SEEG in two thirds of patients (4/6) with initial failed temporal resections revealed expanded SOZ to include the insula. All 13 patients (100%) had a subsequent surgery after SEEG which was either LITT (54%) or surgical resection (46%). After the subsequent surgery, a favorable outcome (Engel class I/II) was achieved by eight patients (62%), while five patients experienced an unfavorable outcome (Engel class III/IV, 38%). Of the six patients with secondary surgical resection, four patients (67%) had favorable outcomes, while of the seven patients with LITT, two patients (29%) had favorable outcomes (Engel I/II). Average follow-up after the subsequent surgery was 37 months ±23 months.
Conclusion
SEEG following initial failed resective epilepsy surgery may help guide next steps at identifying residual epileptogenic cortex and is associated with favorable seizure control outcomes.

Similar content being viewed by others
Abbreviations
- EZ:
-
Epileptogenic zone
- EEG:
-
Electroencephalography
- FCD:
-
Focal cortical dysplasia
- iEEG:
-
Intracranial electroencephalography
- LITT:
-
Laser interstitial thermal therapy
- MCD:
-
Malformation of cortical development
- MRI:
-
Magnetic resonance imaging
- rSOZ:
-
Residual seizure onset zone
- SEEG:
-
Stereo-electroencephalography
- SOZ:
-
Seizure onset zone
- TLE:
-
Temporal lobe epilepsy
References
Harris WB, Brunette-Clement T, Wang A et al (2022) Long-term outcomes of pediatric epilepsy surgery: individual participant data and study level meta-analyses. Seizure Eur J Epilepsy 101:227–236. https://doi.org/10.1016/j.seizure.2022.08.010
Andrews JP, Gummadavelli A, Farooque P et al (2019) Association of seizure spread with surgical failure in epilepsy. JAMA Neurol 76(4):462–469. https://doi.org/10.1001/jamaneurol.2018.4316
Ryvlin P, Kahane P (2005) The hidden causes of surgery-resistant temporal lobe epilepsy: extratemporal or temporal plus? editorial review. Curr Opin Neurol 18(2):125. https://doi.org/10.1097/01.wco.0000162852.22026.6f
Jeha LE, Najm IM, Bingaman WE et al (2006) Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 66(12):1938–1940. https://doi.org/10.1212/01.wnl.0000219810.71010.9b
Barba C, Rheims S, Minotti L et al (2016) Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain 139(2):444–451. https://doi.org/10.1093/brain/awv372
Bartolomei F, Lagarde S, Wendling F et al (2017) Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia 58(7):1131–1147. https://doi.org/10.1111/epi.13791
Yardi R, Morita-Sherman ME, Fitzgerald Z et al (2020) Long-term outcomes of reoperations in epilepsy surgery. Epilepsia 61(3):465–478. https://doi.org/10.1111/epi.16452
Khoo HM, Hall JA, Dubeau F et al (2020) Technical aspects of seeg and its interpretation in the delineation of the epileptogenic zone. Neurol Med Chir (Tokyo) 60(12):565–580. https://doi.org/10.2176/nmc.st.2020-0176
Iida K, Otsubo H (2017) Stereoelectroencephalography: indication and Efficacy. Neurol Med Chir (Tokyo) 57(8):375–385. https://doi.org/10.2176/nmc.ra.2017-0008
Kovac S, Vakharia VN, Scott C, Diehl B (2017) Invasive epilepsy surgery evaluation. Seizure 44:125–136. https://doi.org/10.1016/j.seizure.2016.10.016
Jayakar P, Gotman J, Harvey AS et al (2016) Diagnostic utility of invasive EEG for epilepsy surgery: indications, modalities, and techniques. Epilepsia 57(11):1735–1747. https://doi.org/10.1111/epi.13515
Vaugier L, Lagarde S, McGonigal A et al (2018) The role of stereoelectroencephalography (SEEG) in reevaluation of epilepsy surgery failures. Epilepsy Behav EB 81:86–93. https://doi.org/10.1016/j.yebeh.2018.02.005
Najm I, Lal D, Alonso Vanegas M et al (2022) The ILAE consensus classification of focal cortical dysplasia: an update proposed by an ad hoc task force of the ILAE diagnostic methods commission. Epilepsia 63(8):1899–1919. https://doi.org/10.1111/epi.17301
Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM (1993) Outcome with respect to epileptic seizures. In: Engel J Jr (ed) Surgical treatment of the epilepsies, ed 2. Raven Press, pp 609–621
Kim SK, Wang KC, Hwang YS et al (2008) Epilepsy surgery in children: outcomes and complications. J Neurosurg Pediatr 1(4):277–283. https://doi.org/10.3171/PED/2008/1/4/277
Englot DJ, Han SJ, Rolston JD et al (2014) Epilepsy surgery failure in children: a quantitative and qualitative analysis: clinical article. J Neurosurg Pediatr 14(4):386–395. https://doi.org/10.3171/2014.7.PEDS13658
McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GCA, Briellmann RS, Berkovic SF (2004) Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 127(9):2018–2030. https://doi.org/10.1093/brain/awh221
Wiebe S, Blume WT, Girvin JP, Eliasziw M (2001) A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345(5):311–318. https://doi.org/10.1056/NEJM200108023450501
Harroud A, Bouthillier A, Weil AG, Nguyen DK (2012) Temporal lobe epilepsy surgery failures: a review. Epilepsy Res Treat 2012:201651. https://doi.org/10.1155/2012/201651
Li J, Reiter-Campeau S, Namiranian D et al (2022) Insular involvement in cases of epilepsy surgery failure. Brain Sci 12(2):125. https://doi.org/10.3390/brainsci12020125
Obaid S, Zerouali Y, Nguyen DK (2017) Insular epilepsy: semiology and noninvasive investigations. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 34(4):315–323. https://doi.org/10.1097/WNP.0000000000000396
Cohen NT, Chang P, You X et al (2022) Prevalence and risk factors for pharmacoresistance in children with focal cortical dysplasia-related epilepsy. Neurology 99(18):e2006-2013. https://doi.org/10.1212/WNL.0000000000201033
Holthausen H, Pieper T, Winkler P, Bluemcke I, Kudernatsch M (2014) Electro-clinical-pathological correlations in focal cortical dysplasia (FCD) at young ages. Childs Nerv Syst 30(12):2015–2026. https://doi.org/10.1007/s00381-014-2549-6
Jayalakshmi S, Vooturi S, Vadapalli R, Madigubba S, Panigrahi M (2021) Predictors of surgical outcome in focal cortical dysplasia and its subtypes. J Neurosurg 136(2):512–522. https://doi.org/10.3171/2020.12.JNS203385
Krsek P, Pieper T, Karlmeier A et al (2009) Different presurgical characteristics and seizure outcomes in children with focal cortical dysplasia type I or II. Epilepsia 50(1):125–137. https://doi.org/10.1111/j.1528-1167.2008.01682.x
Cohen NT, Ziobro JM, Depositario-Cabacar DF et al (2020) Measure thrice, cut twice: on the benefit of reoperation for failed pediatric epilepsy surgery. Epilepsy Res 161:106289. https://doi.org/10.1016/j.eplepsyres.2020.106289
Chen H, Modur PN, Barot N et al (2016) Predictors of postoperative seizure recurrence: a longitudinal study of temporal and extratemporal resections. Epilepsy Res Treat 2016:7982494. https://doi.org/10.1155/2016/7982494
Hale AT, Sen S, Haider AS et al (2019) Open resection versus laser interstitial thermal therapy for the treatment of pediatric insular epilepsy. Neurosurgery 85(4):E730–E736. https://doi.org/10.1093/neuros/nyz094
Drane DL, Loring DW, Voets NL et al (2015) Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia 56(1):101–113. https://doi.org/10.1111/epi.12860
Hoppe C, Helmstaedter C (2020) Laser interstitial thermotherapy (LiTT) in pediatric epilepsy surgery. Seizure 77:69–75. https://doi.org/10.1016/j.seizure.2018.12.010
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
We also confirm that each of the authors has contributed significantly to this work and that we have received no financial support for the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wong, G.M., McCray, A., Hom, K. et al. Outcomes of stereoelectroencephalography following failed epilepsy surgery in children. Childs Nerv Syst (2024). https://doi.org/10.1007/s00381-024-06420-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00381-024-06420-w