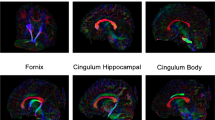
Abstract
Purpose
Nonsyndromic craniosynostosis (NSC) is associated with neurocognitive deficits, and intervention at infancy is standard of care to limit the negative effects of NSC on brain development. In this study, diffusion tensor imaging (DTI) was implemented to investigate white matter microstructure in infants with NSC undergoing cranial vault remodeling, and a comparison was made with white matter development in neurotypical controls.
Methods
Infants presenting with NSC (n = 12) underwent DTI scans before and after cranial vault remodeling. Neurotypical infants (n = 5), age matched to NSC patients at preoperative scans, were compared to preoperative DTI scans. Pre- and postoperative NSC scans were compared in aggregate, and the sagittal synostosis (n = 8) patients were evaluated separately. Finally, neurotypical infants from the University of North Carolina/University of New Mexico Baby Connectome Project (BCP), who underwent DTI scans at timepoints matching the NSC pre- and postoperative DTI scans, were analyzed (n = 9). Trends over the same time period were compared between NSC and BCP scans.
Results
No significant differences were found between preoperative NSC scans and controls. White matter development was more limited in NSC patients than in BCP patients, with microstructural parameters of the corpus body and genu and inferior and superior longitudinal fasciculi consistently lagging behind developmental changes observed in healthy patients.
Conclusion
Infant white matter development appears more limited in NSC patients undergoing cranial vault remodeling relative to that in neurotypical controls. Further investigation is needed to explore these differences and the specific effects of early surgical intervention.




Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Patel A, Terner J, Travieso R, Clune JE, Steinbacher D, Persing JA (2012) On Bernard Sarnat’s 100th birthday: pathology and management of craniosynostosis. J Craniofac Surg 23. https://doi.org/10.1097/SCS.0b013e318240f
Shim KW, Park EK, Kim JS, Kim YO, Kim DS (2016) Neurodevelopmental problems in non-syndromic craniosynostosis. J Korean Neurosurg Soc 59:242–246. https://doi.org/10.3340/jkns.2016.59.3.242
Becker DB, Petersen JD, Kane AA, Cradock MM, Pilgram TK, Marsh JL (2005) Speech, cognitive, and behavioral outcomes in nonsyndromic craniosynostosis. Plast Reconstr Surg 116:400–407
Da Costa AC, Anderson VA, Holmes AD, Lo P, Wray AC, Chong DK, Greensmith AL, Meara JG (2013) Longitudinal study of the neurodevelopmental characteristics of treated and untreated nonsyndromic craniosynostosis in infancy. Child’s Nervous System 29:985–995. https://doi.org/10.1007/s00381-012-2017-0
Chieffo D, Tamburrini G, Massimi L, Di Giovanni S, Giansanti C, Caldarelli M, Di Rocco C (2010) Long-term neuropsychological development in single-suture craniosynostosis treated early: clinical article. J Neurosurg Pediatr 5:232–237. https://doi.org/10.3171/2009.10.PEDS09231
Collett BR, Kapp-Simon KA, Wallace E, Cradock MM, Buono L, Speltz ML (2017) Attention and executive function in children with and without single-suture craniosynostosis. Child Neuropsychol 23:83–98. https://doi.org/10.1080/09297049.2015.1085005
Gabrick KS, Wu RT, Singh A, Persing JA, Alperovich M (2020) Radiographic severity of metopic craniosynostosis correlates with long-term neurocognitive outcomes. Plast Reconstr Surg 145:1241–1248. https://doi.org/10.1097/PRS.0000000000006746
Aldridge K, Collett BR, Wallace ER, Birgfeld C, Austin JR, Yeh R, Feil M, Kapp-Simon KA, Aylward EH, Cunningham ML, Speltz ML (2017) Structural brain differences in school-age children with and without single-suture craniosynostosis. J Neurosurg Pediatr 19:479–489. https://doi.org/10.3171/2016.9.PEDS16107
Cabrejo R, Lacadie C, Sun A, Chuang C, Yang J, Brooks E, Beckett J, Eilbott J, Gabrick K, Steinbacher D, Duncan C, Diluna M, Alperovich M, Pelphrey K, Ventola P, Constable T, Persing JA (2021) Functional network development in sagittal craniosynostosis treated with whole vault cranioplasty. J Craniofac Surg 32:1721–1726. https://doi.org/10.1097/SCS.0000000000007505
Brooks ED, Yang J, Beckett JS, Lacadie C, Scheinost D, Persing S, Zellner EG, Oosting D, Keifer C, Friedman HE, Vander WB, Jou RJ, Sun H, Gary C, Duncan CC, Constable RT, Pelphrey KA, Persing JA (2016) Normalization of brain morphology after surgery in sagittal craniosynostosis. J Neurosurg Pediatr 17:460–468. https://doi.org/10.3171/2015.7.PEDS15221
Fontana SC, Belinger S, Daniels D, Tuttle M, Camarata PJ, Andrews BT (2018) Longitudinal assessment of developmental outcomes in infants undergoing late craniosynostosis repair. J Craniofac Surg 29:25–28. https://doi.org/10.1097/SCS.0000000000004024
Patel A, Yang JF, Hashim PW, Travieso R, Terner J, Mayes LC, Kanev P, Duncan C, Jane J, Pollack I, Losee JE, Bridgett DJ, Persing JA (2014) The impact of age at surgery on long-term neuropsychological outcomes in sagittal craniosynostosis. Plast Reconstr Surg 134:608e–617e. https://doi.org/10.1097/PRS.0000000000000511
O’Donnell LJ, Westin CF (2011) An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 22:185–196
Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL (2009) Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry 66:238–244. https://doi.org/10.1016/j.biopsych.2009.02.025
Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L (2012) Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Dev Med Child Neurol 54:38–43. https://doi.org/10.1111/j.1469-8749.2011.04147.x
Saxena K, Tamm L, Walley A, Simmons A, Rollins N, Chia J, Soares JC, Emslie GJ, Fan X, Huang H (2012) A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. J Child Adolesc Psychopharmacol 22:112–119. https://doi.org/10.1089/cap.2011.0063
Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. https://doi.org/10.3389/fnins.2013.00031
Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, Van Zijl PCM, Mori S (2006) Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29:493–504. https://doi.org/10.1016/J.NEUROIMAGE.2005.08.017
McGraw P, Liang L, Provenzale JM (2012) Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. Am J Roentgenol 179:1515–1522. https://doi.org/10.2214/AJR.179.6.1791515
Schneider JFL, Il’yasov KA, Hennig J, Martin E, (2004) Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology 46:258–266. https://doi.org/10.1007/S00234-003-1154-2/FIGURES/3
Mukherjee P, McKinstry RC (2006) Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am 16:19–43. https://doi.org/10.1016/J.NIC.2005.11.004
Ouyang M, Dubois J, Yu Q, Mukherjee P, Huang H (2019) Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage 185:836–850. https://doi.org/10.1016/J.NEUROIMAGE.2018.04.017
de Planque CA, Gaillard L, Vrooman HA, Li B, Bron EE, van Veelen MLC, Mathijssen IMJ, Dremmen MHG (2022) A diffusion tensor imaging analysis of frontal lobe white matter microstructure in trigonocephaly patients. Pediatr Neurol 131:42–48. https://doi.org/10.1016/j.pediatrneurol.2022.04.003
Florisson JMG, Dudink J, Koning IV, Hop WCJ, Van Veelen MLC, Mathijssen IMJ, Lequin MH (2011) Assessment of white matter microstructural integrity in children with syndromic craniosynostosis: a diffusion-tensor imaging study. Radiology 261:534–541. https://doi.org/10.1148/radiol.11101024
Rijken BFM, Leemans A, Lucas Y, Van Montfort K, Mathijssen IMJ, Lequin MH (2015) Diffusion tensor imaging and fiber tractography in children with craniosynostosis syndromes. Am J Neuroradiol 36:1558–1564. https://doi.org/10.3174/ajnr.A4301
Aghajani M, Veer IM, Van Lang NDJ, Meens PHF, Van Den Bulk BG, Rombouts SARB, Vermeiren RRJM, Van Der Wee NJ (2014) Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med 44:2287–2298. https://doi.org/10.1017/S0033291713003000
Wu RT, Yang JF, Zucconi W, Lacadie C, Swallow MS, Sun AH, Eilbott J, Mayes LC, Steinbacher DM, Pelphrey K, Persing JA (2019) Frustration and emotional regulation in nonsyndromic craniosynostosis: a functional magnetic resonance imaging study. Plast Reconstr Surg 144:1371–1383. https://doi.org/10.1097/PRS.0000000000005850
Vestergaard M, Madsen KS, Baaré WFC, Skimminge A, Rye Ejersbo L, Ramsøy TZ, Gerlach C, Paulson OB, Jernigan TL (2011) White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J Cogn Neurosci 23:2135–2146
Beckett JS, Brooks ED, Lacadie C, Vander WB, Jou RJ, Steinbacher DM, Constable RT, Pelphrey KA, Persing JA (2014) Altered brain connectivity in sagittal craniosynostosis: laboratory investigation. J Neurosurg Pediatr 13:690–698. https://doi.org/10.3171/2014.3.PEDS13516
Brooks ED, Beckett JS, Yang J, Timberlake AT, Sun AH, Chuang C, Persing JA (2018) The etiology of neuronal development in craniosynostosis: a working hypothesis. J Craniofac Surg 29:49–55. https://doi.org/10.1097/SCS.0000000000004040
Sun AH, Eilbott J, Chuang C, Yang JF, Brooks ED, Beckett J, Steinbacher DM, Pelphrey K, Persing JA (2019) An investigation of brain functional connectivity by form of craniosynostosis. J Craniofac Surg 30:1719–1723. https://doi.org/10.1097/SCS.0000000000005537
Funding
Dr. Alperovich receives funding from CTSA Grant Number KL2 TR001862 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH) and consults for Johnson & Johnson and LifeNet Health.
Author information
Authors and Affiliations
Contributions
M.N.A. and M.A. conceived the experiment. M.N.A., K.G.H., J.M.H.I., and N.P. performed patient recruitment and data collection. J.M. planned the analysis with C.L. and with guidance from M.A., M.N.A., and J.A.P. C.L. performed the analysis. J.M. drafted the manuscript with critical input and revision from all authors (M.N.A., C.L., K.G.H., J.M.H.I., N.P., J.A.P., M.A.). M.A. supervised and directed the study.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Yale Institutional Review Board (HIC # 2000030969).
Consent to participate
Informed consent was obtained from legal guardians for all individual participants included in this study.
Competing interests
The authors declare no competing interests.
Disclaimer
The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Research reported in this publication was also supported by the Richard K. Gershon Endowed Medical Student Research Fellowship and Yale School of Medicine Fellowship for Medical Student Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of Richard K. Gershon Endowed Medical Student Research Fellowship and Yale School of Medicine Fellowship for Medical Student Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moscarelli, J., Almeida, M.N., Lacadie, C. et al. A diffusion tensor imaging comparison of white matter development in nonsyndromic craniosynostosis to neurotypical infants. Childs Nerv Syst 40, 1477–1487 (2024). https://doi.org/10.1007/s00381-023-06262-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06262-y