Abstract
Ischemic preconditioning (IPC) describes a phenomenon wherein brief ischemia of the heart induces a potent cardioprotective mechanism against succeeding ischemic insult. Cyclooxygenase-2 (COX-2), a rate-limiting enzyme in prostanoid biosynthesis, is upregulated in the ischemic heart and contributes to IPC. Prostaglandin E2 (PGE2) protects the heart from ischemia–reperfusion (I/R) injury via its receptor subtype EP4. We sought to clarify the role of the PGE2/EP4 system in the late phase of IPC. Mice were subjected to four IPC treatment cycles, consisting of 5 min of occlusion of the left anterior descending coronary artery (LAD). We found that COX-2 mRNA was significantly upregulated in wild-type hearts at 6 h after IPC treatment. Cardiac PGE2 levels at 24 h after IPC treatment were significantly increased in both wild-type mice and mice lacking EP4 (EP4–/–). At 24 h after IPC treatment, I/R injury was induced by 30 min of LAD occlusion followed by 2 h of reperfusion and the cardiac infarct size was determined. The infarct size was significantly reduced by IPC treatment in wild-type mice; a reduction was not observed in EP4–/– mice. AE1-329, an EP4 agonist, significantly reduced infarct size and significantly ameliorated deterioration of cardiac function in wild-type mice subjected to I/R without IPC treatment. Furthermore, AE1-329 significantly enhanced the I/R-induced activation of Akt, a pro-survival kinase. We demonstrated that the PGE2/EP4 system in the heart plays a critical role in the late phase of IPC, partly by augmenting Akt-mediated signaling. These findings clarify the mechanism of IPC and may contribute to the development of therapeutic strategies for ischemic heart disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic preconditioning (IPC) is well documented as a potent cardioprotective phenomenon [1,2,3]. It refers to a brief ischemic episode of the heart that induces a potent cardioprotective mechanism against succeeding ischemic insult. IPC consists of two phases, an early and a late phase [1,2,3]. The early phase develops within minutes of an initial ischemic episode and lasts for 2–4 h [1,2,3]. The late phase begins 12–24 h after the initial ischemic episode and persists for 72–96 h [1,2,3]. Because the late phase of IPC protects the heart from both myocardial infarction and from stunning for a substantial period of time [4, 5], it has potential clinical relevance. Many previous studies have focused on the complex mechanisms underlying IPC; however, the mechanisms remain to be clarified in detail [6].
The current consensus is that the early phase of IPC is mediated by the activation of a preexisting signaling cascade [7], whereas the late phase results from the synthesis of cardioprotective mediators. Recent studies have elucidated that cyclooxygenase-2 (COX-2), a rate-limiting enzyme for the synthesis of prostanoids, is crucial for the late phase of IPC [8, 9] and atherosclerotic plaque stabilization [10]. Accordingly, the late phase of IPC is abrogated by COX-2-selective inhibitors, such as NS-398 and celecoxib [11]. Upregulation of COX-2 during IPC results in increased synthesis of cardioprotective prostaglandins (PG), such as prostaglandins I2 and E2 (PGE2) [8, 9, 11]. However, it remains to be determined which type(s) of PG participate in the cardioprotection afforded by COX-2 in the late phase of IPC.
PGE2 exerts various actions through each of its receptor subtypes (EP1, EP2, EP3, and EP4) [12]. It has been reported that EP4 mRNA is highly expressed in the hearts of several species, including mouse and human, which suggests that EP4 may have some role in the heart. Indeed, studies have shown the upregulation of EP4 expression levels in mouse models of myocardial infarction [13]. Importantly, our previous study demonstrated that PGE2 exerted a potent cardioprotective effect via EP4 in ischemia/reperfusion (I/R) injury [14]. In mice lacking EP4 (EP4–/–), I/R injury was abrogated significantly in an in vivo I/R model and in an ex vivo perfused heart model. This indicated that PGE2’s cardioprotective effect was mediated within the heart through the PGE2/EP4 system.
Furthermore, an EP4-specific agonist has been reported to impart a cardioprotective effect by suppressing the expression of macrophage chemoattractant protein-1, thus inhibiting the infiltration of macrophages into the ischemic area [15]. This indicates that EP4 in macrophages also can participate in the cardioprotective mechanisms of the PGE2/EP4 system. While a recent report showed that PGE2/EP4 activation ameliorates hepatic I/R injury via the ERK1/2/glycogen synthase kinase (GSK) 3β pathway [16], the role of the PGE2/EP4 system in the IPC remains to be clarified.
We hypothesized that PGE2, derived from COX-2, is upregulated by brief ischemic stress and contributes to the late phase of IPC via EP4. To test this hypothesis, we examined I/R injury in an EP4–/– mouse model of IPC. Using a novel EP4-specific agonist, AE1-329, we sought a mechanistic explanation for the cardioprotective function of the PGE2/EP4 system.
Materials and methods
Mice
The details of the breeding and maintenance of animals used in the present study were previously reported [17]. Most EP4–/– mice die postnatally as a result of patent ductus arteriosus or do not survive in the C57BL/6 background. Therefore, F2 progenies of surviving EP4–/– mice and their wild-type litter mates were independently maintained in a mixed genetic background of 129/Ola and C57BL/6 [18]. All experiments were performed using 7–12-week-old male mice per the guidelines of Japan’s Act on Welfare and Management of Animals and were approved by the Asahikawa Medical University Committee on Animal Research.
IPC and I/R procedures
The study mice were anesthetized with pentobarbital (60 mg/kg body weight, intraperitoneally) and secured in a supine position with the upper and lower extremities held on a heated table under a two-lead electrocardiogram (ECG) monitoring device. Tracheal intubation with a blunt 20-gauge polyethylene tube (Terumo, Tokyo, Japan) was performed under direct visualization of the tube through the tracheal wall at the upper border of the thyroid cartilage, which was surgically exposed. The tracheal tube was connected to a mechanical ventilator (SN-480-7; Shinano, Tokyo, Japan) and the mice were ventilated using a volume-controlled ventilation mode (0.8 ml room air/breath at 110 breaths/min). After the left anterior thoracotomy, the heart was exposed and the pericardium dissected; the dissection table was tilted up and to the left to visually identify the left coronary artery. An 8–0 nylon suture was passed underneath the left anterior descending coronary artery (LAD) at a position 1 mm from the tip of the left auricle. A small length of polyethylene tube with a blunt edge (size 3; Hibiki, Tokyo, Japan) was threaded by two lines of suture and mounted vertically on the LAD, with a piece of rubber (1 g) attached to each end of the suture. The LAD was occluded by a suture supporting rubber weights and was reopened by manually releasing the weight loading. To confirm that the LAD was successfully occluded, the myocardium was checked for color change (from brick red to pale). In addition, ECG observations showed prolonged QRS duration, enlarged QRS voltage, and ST elevation after successful occlusion [19]. On Day 1, mice in the IPC group underwent the IPC treatment, consisting of four 5-min cycles of occlusion followed by 5 min of reperfusion of the LAD. Mice in the IPC sham group underwent the same surgery, apart from IPC treatment. On Day 2, 24 h after IPC treatment, the mice were subjected to 30 min of LAD occlusion followed by 2 h of reperfusion. To examine the effects of the EP4 agonist, AE1-329 [20], I/R injury was induced by occluding the LAD for 30 min, without IPC treatment, followed by indicated times of reperfusion. AE1-329 (30 μg/kg) was injected subcutaneously 30 min before LAD occlusion.
Reverse transcription polymerase chain reaction analysis for COX-2 mRNA
The hearts of wild-type mice were excised 6 h after IPC or sham treatment and total RNA was prepared from ischemic and nonischemic areas using Isogen (Nippon Gene, Toyama, Japan). Total RNA (2 μg) was reverse-transcribed, as previously reported (10). The resulting cDNA was amplified by polymerase chain reaction (PCR) using primer sets corresponding to COX-2 mRNA, as follows:
sense 5′-ACACTCTATCACTGGCACCC-3′
antisense 5′-GGACGAGGTTTTTCCAC-CAG-3′
The quantity of PCR product was determined by real-time PCR analysis using Lightcycler apparatus (Idaho Technology, Idaho Falls, ID, USA) and DNA Master SYBR Green I (Roche Molecular Biochemicals, Mannheim, Germany) as previously described [20]. The values for the ischemic areas were expressed in relation to the nonischemic areas.
PGE2 enzyme immunoassay
Hearts were excised immediately or 24 h after IPC treatment. Tissue samples were prepared from ischemic (anterior left ventricular wall) and nonischemic (posterior left ventricular wall) areas by homogenization in 0.1 M phosphate buffer containing 1 mM EDTA and 10 μM indomethacin. Prostaglandins were pre-extracted from tissue samples using silica-based octadecylsilane reverse-phase columns. The levels of PGE2 in the samples were determined using an enzyme-linked immunoassay kit (Cayman Chemicals, Ann Arbor, MI, USA), according to the manufacturer’s instructions.
Determination of the area at risk (AAR) and myocardial infarct size
After 30 min of LAD occlusion (with or without IPC treatment) and following reperfusion of 2 h, the size of the AAR and the infarct size were determined using a double-staining technique [21]. Briefly, the right carotid artery was exposed via blunt dissection of the paratracheal muscles, and then cannulated with a catheter. AAR was determined by retrograde injection of 5.0% Evans Blue dye (300 μl) through the catheter while the LAD was occluded. By this procedure, all cardiac tissue except the AAR was stained blue. After the heart was excised and washed in ice-cold phosphate-buffered saline (PBS), it was frozen at − 80 °C for 5 min and cut into five slices. The slices were incubated with 1.0% triphenyltetrazolium chloride (TTC) at 37 °C for 5 min, followed by overnight immersion in PBS at 4 °C. Thus, the infarcted area was demarcated as a pale-to-white area, while viable tissue was stained red. AAR and infarct size were determined via planimetry using Photoshop 7.0 (Adobe, San Jose, CA, USA). The infarct size was calculated as ratio of the infarct (pale-to-white area) to AAR (all cardiac tissue except blue areas) since the perfusion territory of LAD was different in each mouse [21].
Echocardiographic examination of the cardiac function
Echocardiography was performed before the I/R procedure, and after 2 h of reperfusion following 30 min of LAD occlusion, using a Vevo 660 machine (Primetech; VisualSonics, Toronto, Canada) with a 35-MHz probe. First, a B-mode image of the left ventricle (LV) was obtained in the short-axis view at the level of the papillary muscles. Then, end-diastolic and -systolic LV dimensions were measured from the M-mode tracings. LV ejection fraction (LVEF) was calculated using the equation: LVEF = (LV diastolic volume − LV systolic volume)/LV diastolic volume) × 100, where LV diastolic and systolic volumes indicate left ventricular diastolic and systolic volumes, respectively.
Western blotting analysis for Akt
Hearts were excised after 15 min of reperfusion following 30 min of LAD occlusion without IPC treatment. The ischemic area was harvested, frozen in liquid nitrogen, and stored at − 80 °C until use. For detection of Ser473-phospholylated Akt (p-Akt) and Akt, the samples were homogenized in lysis buffer (20 mM Tris, 1 mM EDTA, 1 mM DTT, 1% Triton X-100, 2 mM Na3VO4, 2 mM NaF, 10 mM sodium pyrophosphate, protease inhibitor cocktail; pH 7.5). After centrifugation for 10 min at 12,000 rpm, protein concentrations in the supernatant were determined using the bicinchoninic acid (BCA) Protein Assay Kit (Pierce; Thermo Fisher Scientific, Waltham, MA, USA). The sample (40 μg protein) was electrophoresed using sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride transfer membrane (Millipore, Billerica, MA, USA) using a semidry transfer system (ATTO, Tokyo, Japan). After a blocking procedure using 5% nonfat dried milk for 1 h at room temperature, the membranes were incubated with the antibodies against p-Akt or Akt (×1000; Cell Signaling Technology) at 4 °C overnight. P-Akt and Akt were detected using horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Chicago, IL, USA) and an enhanced chemiluminescence reagent. Densitometry of the bands was analyzed using Photoshop as previously described [20].
Statistical analyses
All data are expressed as mean ± standard error. The data were analyzed using the Student’s t test for unpaired samples. P values of < 0.05 were considered statistically significant.
Results
Augmented COX-2 mRNA expression and PGE2 production in the heart after IPC treatment
The expression level of COX-2 mRNA in the heart of sham-operated wild-type mice was low and barely detectable. However, after IPC treatment, the expression level of COX-2 mRNA in the ischemic area increased remarkably at 6 h (Fig. 1), which indicates that the production of cardioprotective prostanoids (such as PGE2) increases in the heart subjected to IPC treatment. Immediately after the IPC treatment, PGE2 levels in the ischemic area were similar to those of the nonischemic control areas (Fig. 2), which indicated that there was no increase in PGE2 production at that time point. However, the PGE2 level in the ischemic area increased significantly at 24 h after IPC treatment, compared with that of the nonischemic control area, in both wild-type and EP4–/– hearts to a similar degree (Fig. 2), indicating that PGE2 may play a role in the late phase of IPC. PGE2 levels in the nonischemic areas at 24 h after IPC treatment did not differ significantly between wild-type and EP4–/– hearts (5.24 ± 0.73 pg/mg [n = 9] and 4.10 ± 0.38 pg/mg [n = 6], respectively).
Upregulation of COX-2 mRNA after the IPC treatment. Wild-type hearts received a brief ischemic stress from four 5-min cycles of LAD occlusion, followed by 5 min of reperfusion. At 6 h after IPC treatment, tissue samples were prepared from ischemic and nonischemic areas of the heart, and then examined for the expression of COX-2 mRNA, using A RT-PCR or B quantitative RT-PCR. The values were expressed as percentages of the nonischemic area, representing the mean values from two independent experiments. n = 3. *P < 0.05 vs. sham-operated
The late phase of IPC observed in wild-type hearts disappears in EP4 –/– hearts
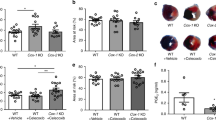
To determine the role of PGE2 via EP4 in late-phase IPC, we performed the I/R procedure after IPC treatment in wild-type and EP4–/– mice (Fig. 3A). In wild-type mice, the infarct size of the heart after IPC treatment was significantly smaller than in sham-operated mice (Fig. 3B, C), indicating that the late phase of IPC works effectively in wild-type mice. In contrast, there was no significant difference in the infarct size between the IPC-treated and sham-operated groups in EP4–/– mice, indicating that the late phase of IPC disappeared completely in EP4–/– mice. In sham-operated groups, there were no significant differences in both infarct size and AAR between wild-type and EP4–/– mice (Fig. 3C). These results clearly show that the PGE2/EP4 system plays a critical role in the late phase of IPC.
IPC treatment reduces the infarct size in wild-type hearts but not in EP4–/– hearts. A The experimental protocol for the IPC and I/R procedures (O: LAD occlusion; R: reperfusion). B Representative photomicrographs of LV sections from wild-type and EP4–/– mice after 2 h of reperfusion following 30 min of LAD occlusion. Tissues that were stained blue (Evans Blue dye) represent nonischemic areas; tissues stained red (TTC) within the ischemic area are live tissues. Unstained tissues appear pale-to-white and represent necrotic myocardium. C Cardiac infarct size and AAR were measured in wild-type and EP4–/– hearts. The values presenting the infarct size are expressed as percentages of the AAR. n = 4–5. *P < 0.01 vs. sham-operated group
AE1-329, an EP4 agonist, reduces the infarct size of wild-type hearts after the I/R procedure without IPC treatment
To clarify whether insufficient production of PGE2 in the heart was responsible for the lack of significant difference in infarct sizes between sham-operated wild-type and EP4–/– hearts, we used AE1-329 to activate EP4 in wild-type hearts that did not receive IPC treatment. AE1-329 (30 mg/kg) was injected 30 min before the 30-min LAD occlusion and the infarct size was determined after 2 h of reperfusion. AE1-329 significantly reduced the infarct size in wild-type mice (Fig. 4), whereas no such effect was observed in EP4–/– mice (data not shown). In contrast to the efficient activation of EP4 and the resultant reduction in the infarct size in the heart with the IPC treatment (Fig. 3C), this result indicates that the endogenous production of PGE2 in hearts without IPC treatment was insufficient to activate EP4, at least during the 2 h of the I/R procedure. AE1-329 did not significantly affect blood pressure (data not shown).
AE1-329, an EP4 agonist, reduces infarct size and ameliorates impaired function in wild-type hearts after I/R. A Representative photomicrographs of LV sections from control and AE1-329-pretreated mice after 2 h of reperfusion following 30 min of LAD occlusion. B Cardiac infarct size and AAR were measured. The values presenting the infarct size are expressed as percentages of the AAR. n = 6. *P < 0.01 vs. control. C Representative M-mode tracings of the LV from control and AE1-329-pretreated mice after 2 h of reperfusion following 30 min of LAD occlusion. D LVEF was measured. n = 5–6. *P < 0.05 vs. control
We used echocardiography to examine the effects of AE1-329 pretreatment on the function of wild-type hearts at 2 h of reperfusion following 30 min of LAD occlusion. In the hearts pretreated with AE1-329, movement of the anterior wall was well retained, compared with that of control hearts (Fig. 4C). Additionally, AE1-329 significantly prevented a reduction in the LVEF after the I/R procedure (Fig. 4D), which indicated that the activation of EP4 protected the heart from I/R injury, both histologically and functionally.
AE1-329 enhances I/R-induced activation of Akt
To further investigate the cardioprotective mechanism of the PGE2/EP4 system, we examined the activation of Akt, a pro-survival serine/threonine kinase. We measured the levels of phosphorylated Akt (p-Akt), an activated form of Akt. Although AE1-329 alone did not alter the level of p-Akt, it significantly enhanced the I/R-induced increase in p-Akt level in wild-type hearts (Fig. 5), the enhancement of Akt activation by AE1-329 was not observed in EP4–/– hearts (Fig. 5). Both the molecular weight of p-Akt and Akt is 60 kD, suggesting that the upper bands in p-Akt were non-specific. The levels of total Akt were not different between wild-type and EP4–/– hearts, irrespective of the presence or absence of AE1-329. These results suggested that Akt signaling underlies the cardioprotective mechanism of the PGE2/EP4 system in I/R. Meanwhile, I/R treatment increased p-Akt/Akt ratio in EP4−/− mice, suggesting that Akt activation might be increased via pathways other than PGE2/EP4 system.
AE1-329 enhances the I/R-induced activation of Akt. Akt and p-Akt levels in wild-type and EP4–/– hearts were determined at 15 min of reperfusion following 30 min of LAD occlusion. A A representative result of Western blotting showing the levels of Akt and p-Akt. B Effects of AE1-329 on the activation of Akt after I/R. Molecular weights of both p-Akt and Akt are 60 kD. The ratio of p-Akt to Akt is expressed as a percentage of that of the nonischemic wild-type control heart. n = 3–5. *P < 0.05 vs. respective AE1-329-untreated control. **P < 0.01
Discussion
Our results show that the PGE2/EP4 system plays a critical role in the heart in the late phase of IPC, partly by augmenting Akt-mediated signaling. IPC treatment significantly increased the cardiac expression level of COX-2 mRNA and the production of PGE2, thus inducing a potent late phase of IPC in wild-type hearts. However, in EP4–/– hearts, the late phase of IPC did not establish, which indicates that the PGE2/EP4 system critically mediates the late phase of IPC. We have previously reported that PGE2 protects the heart from I/R injury via EP4. We found that the infarct size was significantly larger in EP4 hearts than that in wild-type hearts at 24 h of reperfusion following 1 h of LAD occlusion. However, the present study found no significant difference in the infarct size between sham-operated wild-type and EP4 mice at 2 h of reperfusion following 30 min of LAD occlusion. This suggests that PGE2 production in the heart, without the IPC treatment, was insufficient to activate EP4 at this time point; effective activation of EP4 and a resultant decrease in the infarct size were observed in wild-type hearts with IPC treatment. Activation of EP4 by exogenous AE1-329 significantly reduced the infarct size and rescued the functional deterioration induced by I/R at the same time point. Taken together, these results support a hypothesis that PGE2 originating from COX-2, upregulated by a brief ischemic stress, contributed critically, via EP4, to the late phase of IPC. Several reports have demonstrated that EP4 signaling provides protection from myocardial I/R injury [14, 15, 22]. This is the first study to demonstrate that COX2 upregulation by IPC attenuated ischemic injury through PGE2/EP4 signaling. Further studies are warranted to clarify the role of PGE2/EP4 signaling in the late phase of IPC.
Although a cardioprotective role of the PGE2/EP4 system in I/R injury has been reported previously [14], its precise mechanism remained to be clarified. During myocardial I/R injury, EP2 and EP4 play a cardioprotective role after ischemia through the activation of the cyclic AMP/protein kinase A signaling pathway [23]. It is known that phosphatidylinositol 3-kinase (PI3-K)/Akt signaling plays an important antiapoptotic and cardioprotective role in cardiac I/R injury [6, 24,25,26]. Indeed several agents capable of protecting the heart from I/R injury activate PI3-K/Akt signaling when given at reperfusion, such as insulin [27], erythropoietin [28], and bradykinin [29]. A recent study demonstrated that miR-486-5p, a cardioprotective microRNA that can activate the phosphotidylinositol 3-kinase/Akt signaling pathway, was dysregulated in rat models of acute myocardial infarction and in patients with acute myocardial infarction [30].
It has been reported that PGE2 activates Akt via EP4 in several types of cells, such as human embryonic kidney cells expressing EP4 [31], glomerular epithelial cells [32], and human lung carcinoma cells [33]. This suggests the possibility that the PGE2/EP4 system is also able to activate Akt during cardiac I/R, thus protecting the heart. In the present study, AE1-329 significantly augmented the I/R-induced activation of Akt in wild-type hearts, while no such effect was observed in EP4–/– hearts. This suggests that Akt signaling underlies the cardioprotective mechanism of the PGE2/EP4 system in I/R. Additionally, several studies demonstrated the utility of EP4 as a promising therapeutic target in cardiac diseases including not only ischemic heart disease but also inflammatory heart disease [34] and cardiac hypertrophy [35]. Further investigations are necessary to extend the clinical benefits of EP4 agonists in cardiac disease.
This study has several limitations. We did not evaluate cellular necrosis and apoptosis although the signals downstream of the phosphotidylinositol 3-kinase/Akt pathway confers necrosis and apoptosis. Further studies should be considered to clarify the molecular mechanism underlying the cardioprotective role of the EP4/Akt pathway.
In conclusion, we demonstrated that the PGE2/EP4 system in the heart plays a critical role in the late phase of IPC, partly by augmenting Akt-mediated signaling. Our findings clarify the mechanism of IPC and could contribute to the development of therapeutic strategies for ischemic heart disease.
References
Bolli R (2007) Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292:H19-27
Kloner RA, Jennings RB (2001) Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation 104:2981–2989
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Tang XL, Qiu Y, Park SW, Sun JZ, Kalya A, Bolli R (1996) Time course of late preconditioning against myocardial stunning in conscious pigs. Circ Res 79:424–434
Takano H, Manchikalapudi S, Tang XL, Qiu Y, Rizvi A, Jadoon AK, Zhang Q, Bolli R (1998) Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation 98:441–449
Downey JM, Davis AM, Cohen MV (2007) Signaling pathways in ischemic preconditioning. Heart Fail Rev 12:181–188
Zheng J, Chen P, Zhong J, Cheng Y, Chen H, He Y, Chen C (2021) HIF-1alpha in myocardial ischemia-reperfusion injury (Review). Mol Med Rep 23:352
Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, Bolli R (2002) Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res 90:602–608
Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R (2003) Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol 35:525–537
Zhang K, Kong J, Liu B, Meng X (2020) Regulatory T cells suppress the expression of COX-2 in vulnerable plaque. Heart Vessels 35:278–283
Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B (2002) Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res 55:506–519
Narumiya S, Sugimoto Y, Ushikubi F (1999) Prostanoid receptors: structures, properties, and functions. Physiol Rev 79:1193–1226
Gu X, Xu J, Zhu L, Bryson T, Yang XP, Peterson E, Harding P (2016) Prostaglandin E2 reduces cardiac contractility via EP3 receptor. Circ Heart Fail 9:e003291
Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F (2004) Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation 109:2462–2468
Hishikari K, Suzuki J, Ogawa M, Isobe K, Takahashi T, Onishi M, Takayama K, Isobe M (2009) Pharmacological activation of the prostaglandin E-2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc Res 81:123–132
Cai LL, Xu HT, Wang QL, Zhang YQ, Chen W, Zheng DY, Liu F, Yuan HB, Li YH, Fu HL (2020) EP4 activation ameliorates liver ischemia/reperfusion injury via ERK1/2GSK3betadependent MPTP inhibition. Int J Mol Med 45:1825–1837
Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, Tanaka T, Yoshida N, Narumiya S, Ichikawa A (1998) Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun 246:7–12
Fujino T, Nakagawa N, Yuhki K, Hara A, Yamada T, Takayama K, Kuriyama S, Hosoki Y, Takahata O, Taniguchi T, Fukuzawa J, Hasebe N, Kikuchi K, Narumiya S, Ushikubi F (2004) Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I-2 receptor IP. J Clin Invest 114:805–812
Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK (2006) Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol 291:H2533-2540
Nakagawa N, Yuhki K, Kawabe J, Fujino T, Takahata O, Kabara M, Abe K, Kojima F, Kashiwagi H, Hasebe N, Kikuchi K, Sugimoto Y, Narumiya S, Ushikubi F (2012) The intrinsic prostaglandin E2-EP4 system of the renal tubular epithelium limits the development of tubulointerstitial fibrosis in mice. Kidney Int 82:158–171
Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM (1995) Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269:H2147-2154
Xiao CY, Hara A, Yuhki K, Fujino T, Ma H, Okada Y, Takahata O, Yamada T, Murata T, Narumiya S, Ushikubi F (2001) Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation 104:2210–2215
Birkenmeier K, Janke I, Schunck WH, Trimpert C, Krieg T, Landsberger M, Volker U, Felix SB, Staudt A (2008) Prostaglandin receptors mediate effects of substances released from ischaemic rat hearts on non-ischaemic cardiomyocytes. Eur J Clin Invest 38:902–909
Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101:660–667
Miao W, Luo Z, Kitsis RN, Walsh K (2000) Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J Mol Cell Cardiol 32:2397–2402
Hausenloy DJ, Yellon DM (2006) Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 70:240–253
Baines CP, Wang L, Cohen MV, Downey JM (1999) Myocardial protection by insulin is dependent on phospatidylinositol 3-kinase but not protein kinase C or KATP channels in the isolated rabbit heart. Basic Res Cardiol 94:188–198
Cai Z, Semenza GL (2004) Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation 109:2050–2053
Yang XM, Krieg T, Cui L, Downey JM, Cohen MV (2004) NECA and bradykinin at reperfusion reduce infarction in rabbit hearts by signaling through PI3K, ERK, and NO. J Mol Cell Cardiol 36:411–421
Xu L, Tian L, Yan Z, Wang J, Xue T, Sun Q (2022) Diagnostic and prognostic value of miR-486-5p, miR-451a, miR-21-5p and monocyte to high-density lipoprotein cholesterol ratio in patients with acute myocardial infarction. Heart Vessels. https://doi.org/10.1007/s00380-00022-02172-00382
Fujino H, West KA, Regan JW (2002) Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E-2. J Biol Chem 277:2614–2619
Aoudjit L, Potapov A, Takano T (2006) Prostaglandin E2 promotes cell survival of glomerular epithelial cells via the EP4 receptor. Am J Physiol Renal Physiol 290:F1534-1542
Zheng Y, Ritzenthaler JD, Sun X, Roman J, Han S (2009) Prostaglandin E2 stimulates human lung carcinoma cell growth through induction of integrin-linked kinase: the involvement of EP4 and Sp1. Cancer Res 69:896–904
Takakuma A, Nishii M, Valaperti A, Hiraga H, Saji R, Sakai K, Matsumura R, Miyata Y, Oba N, Nunose F, Ogawa F, Tamura K, Takeuchi I (2021) Prostaglandin-E2 receptor-4 stimulant rescues cardiac malfunction during myocarditis and protects the heart from adverse ventricular remodeling after myocarditis. Sci Rep 11:20961
Wang Q, Oka T, Yamagami K, Lee JK, Akazawa H, Naito AT, Yasui T, Ishizu T, Nakaoka Y, Sakata Y, Komuro I (2017) An EP4 receptor agonist inhibits cardiac fibrosis through activation of PKA signaling in hypertrophied heart. Int Heart J 58:107–114
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanno, T., Nakagawa, N., Aonuma, T. et al. Prostaglandin E2 mediates the late phase of ischemic preconditioning in the heart via its receptor subtype EP4. Heart Vessels 38, 606–613 (2023). https://doi.org/10.1007/s00380-022-02219-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-022-02219-4