Abstract
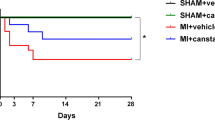
Midkine (MK), a heparin-binding growth factor, has been shown to prevent cardiac remodeling after ischemic injury through its anti-apoptotic effect. Cell apoptosis is central to the pathophysiology of cardiac remodeling in congestive heart failure (CHF) of ischemic as well as non-ischemic origin. We hypothesized that MK exerts the anti-apoptotic cardioprotective effect in CHF of non-ischemic etiology. MK protein or vehicle (normal saline) was subcutaneously administered in tachycardia-induced CHF rabbits (right ventricular pacing, 350 beats/min, 4 weeks). The vehicle-treated rabbits (n = 19, control) demonstrated severe CHF and high mortality rate, whereas MK (n = 16) demonstrated a well-compensated state and a lower mortality rate. In echocardiography, left ventricular (LV) end-diastolic dimension decreased in MK versus control, whereas LV systolic function increased. In histological analysis (picrosirius red staining), MK decreased collagen deposition area compared with control. TUNEL staining showed that MK prevented cell apoptosis and minimized myocyte loss in the CHF rabbit ventricle, associated with activation of PI3-K/Akt signaling, producing a parallel decrease of Bax/Bcl-2 ratio. MK prevented progression of cardiac remodeling in the CHF rabbit, likely by activation of anti-apoptotic signaling. Exogenous MK application might be a novel therapeutic strategy for CHF due to non-ischemic origin.







Similar content being viewed by others
References
Muramatsu T (2002) Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 132(3):359–371
Muramatsu T (2011) Midkine: a promising molecule for drug development to treat diseases of the central nervous system. Curr Pharm Des 17(5):410–423
Horiba M, Kadomatsu K, Yasui K, Lee JK, Takenaka H, Sumida A, Kamiya K, Chen S, Sakuma S, Muramatsu T, Kodama I (2006) Midkine plays a protective role against cardiac ischemia/reperfusion injury through a reduction of apoptotic reaction. Circulation 114(16):1713–1720
Takenaka H, Horiba M, Ishiguro H, Sumida A, Hojo M, Usui A, Akita T, Sakuma S, Ueda Y, Kodama I, Kadomatsu K (2009) Midkine prevents ventricular remodeling and improves long-term survival after myocardial infarction. Am J Physiol Heart Circ Physiol 296(2):H462–H469
Sumida A, Horiba M, Ishiguro H, Takenaka H, Ueda N, Ooboshi H, Opthof T, Kadomatsu K, Kodama I (2010) Midkine gene transfer after myocardial infarction in rats prevents remodelling and ameliorates cardiac dysfunction. Cardiovasc Res 86(1):113–121
Ishiguro H, Horiba M, Takenaka H, Sumida A, Opthof T, Ishiguro YS, Kadomatsu K, Murohara T, Kodama I (2011) A single intracoronary injection of midkine reduces ischemia/reperfusion injury in swine hearts: a novel therapeutic approach for acute coronary syndrome. Front Physiol 2:27
Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA (1996) Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335(16):1182–1189
Saraste A, Pulkki K, Kallajoki M, Heikkila P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM (1999) Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest 29(5):380–386
Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN (2003) A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest 111(10):1497–1504
Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P (1997) Apoptosis in the failing human heart. N Engl J Med 336(16):1131–1141
Harada M, Tsuji Y, Ishiguro YS, Takanari H, Okuno Y, Inden Y, Honjo H, Lee JK, Murohara T, Sakuma I, Kamiya K, Kodama I (2011) Rate-dependent shortening of action potential duration increases ventricular vulnerability in failing rabbit heart. Am J Physiol Heart Circ Physiol 300(2):H565–H573
Narita H, Chen S, Komori K, Kadomatsu K (2008) Midkine is expressed by infiltrating macrophages in in-stent restenosis in hypercholesterolemic rabbits. J Vasc Surg 47(6):1322–1329
Horiba M, Kadomatsu K, Nakamura E, Muramatsu H, Ikematsu S, Sakuma S, Hayashi K, Yuzawa Y, Matsuo S, Kuzuya M, Kaname T, Hirai M, Saito H, Muramatsu T (2000) Neointima formation in a restenosis model is suppressed in Midkine-deficient mice. J Clin Invest 105(4):489–495
Banno H, Takei Y, Muramatsu T, Komori K, Kadomatsu K (2006) Controlled release of small interfering RNA targeting midkine attenuates intimal hyperplasia in vein grafts. J Vasc Surg 44(3):633–641
Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL (1994) Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94(4):1621–1628
Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P (1996) Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 74(1):86–107
Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P (1996) Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol 271(3 Pt 2):H1215–H1228
Liu Y, Cigola E, Cheng W, Kajstura J, Olivetti G, Hintze TH, Anversa P (1995) Myocyte nuclear mitotic division and programmed myocyte cell death characterize the cardiac myopathy induced by rapid ventricular pacing in dogs. Lab Invest 73(6):771–787
Sharov VG, Sabbah HN, Shimoyama H, Goussev AV, Lesch M, Goldstein S (1996) Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol 148(1):141–149
Weckbach LT, Muramatsu T, Walzog B (2011) Midkine in inflammation. Scientific World J 11:2491–2505
Hobo A, Yuzawa Y, Kosugi T, Kato N, Asai N, Sato W, Maruyama S, Ito Y, Konori H, Ikematsu S, Nishiyama A, Matsuo S, Kadomatsu K (2009) The growth factor midkine regulates the renin-angiotensin system in mice. J Clin Invest 119(6):1616–1625
Fujita S, Shimojo N, Terasaki F, Otsuka K, Hosotani N, Kohda Y, Tanaka T, Nishioka T, Yoshida T, Hiroe M, Kitaura Y, Ishizaka N, Imanaka-Yoshida K (2013) Atrial natriuretic peptide exerts protective action against angiotensin II-induced cardiac remodeling by attenuating inflammation via endothelin-1/endothelin receptor A cascade. Heart Vessels 28(5):646–657
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase Akt pathway in human cancer. Nat Rev Cancer 2(7):489–501
Sebolt-Leopold JS, Herrera R (2004) Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 4(12):937–947
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Baraldi PG, Borea PA (2006) Modulation of the Akt/Ras/Raf/Mek/Erk pathway by A3 adenosine receptor. Purinergic Signal 2(4):627–632
Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M (2002) Regulation of Raf-Akt cross-talk. J Biol Chem 277(34):31099–31106
Harada M, Luo X, Qi XY, Tadevosyan A, Maguy A, Ordog B, Ledoux J, Kato T, Naud P, Voigt N, Shi Y, Kamiya K, Murohara T, Kodama I, Tardif JC, Shotten U, Van Wagoner DR, Dobrev D, Nattel S (2012) Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126(17):2051–2064
Olson ER, Shamhart PE, Naugle JE, Meszaros JG (2008) Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase C-delta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension 51(3):704–711
Yamamoto Y, Osanai T, Nishizaki F, Sukekawa T, Izumiyama K, Sagara S, Okumura K (2012) Matrix metalloprotein-9 actiavtion under cell-to-cell interaction between endothelial cells and monocytes: possible role of hypoxia and tumor necrosis factor-α. Heart Vessel 27(6):539–546
Wang W, Peng Y, Wang Y, Zhao X, Yuan Z (2009) Anti-apoptotic effect of heat shock protein 90 on hypoxia-mediated cardiomyocyte damage is mediated via the phospahtidylinositol 3-kinase/AKT pathway. Clin Exp Pharmacol Physiol 36(9):899–903
Kapustian LL, Vigontina OA, Rozhko OT, Ryabenko DV, Michowski W, Lesniak W, Filipek A, Kroupskaya IV, Sidorik LL (2013) Hsp90 and its co-chaperone, Sgt1, as autoantigens in dilated cardiomyopathy. Heart Vessel 28(1):114–119
Kitahara T, Shishido T, Suzuki S, Katoh S, Sasaki T, Ishino M, Nitobe J, Miyamoto T, Miyashita T, Watanabe T, Takeishi Y, Kubota I (2010) Serum midkine as a predictor of cardiac events in patients with chronic heart failure. J Cardiac Fail 16(4):308–313
Netsu S, Shishido T, Kitahara T, Honda Y, Funayama A, Narui T, Kadowaki S, Takahashi H, Miyamoto T, Arimoto T, Nishiyama S, Watanabe T, Woo CH, Takeishi Y, Kubota I (2013) Midkine exacerbates pressure overload-induced cardiac remodeling. Biochem Biophys Res Commun 443(1):205–210
Acknowledgments
We thank Drs. Yukiomi Tsuji and Arihiro Sumida for technical advice and assistance in animal experiments. This work was supported by the Ministry of Education, Culture, Sports, Sciences and Technology, Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harada, M., Hojo, M., Kamiya, K. et al. Exogenous midkine administration prevents cardiac remodeling in pacing-induced congestive heart failure of rabbits. Heart Vessels 31, 96–104 (2016). https://doi.org/10.1007/s00380-014-0569-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-014-0569-5