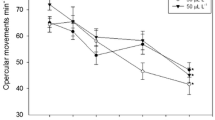
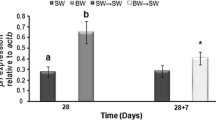
Abstract
Commercial aquaculture production of channel catfish (Ictalurus punctatus) occurs in shallow ponds with daily cycling of dissolved oxygen concentration ranging from supersaturation to severe hypoxia. Once daily minimum dissolved oxygen concentration falls below 3.0 mg O2/L, channel catfish have a reduced appetite, leading to reduced growth rates. In other fishes, upregulation of the neuropeptides corticotropin-releasing factor (CRF) and urotensin I (UI) have been implicated as initiating the mechanism responsible for decreasing appetite once an environmental stressor is detected. Channel catfish maintained at 27 °C in aquaria were subjected to varying durations and patterns of hypoxia (1.75 ± 0.07 mg O2/L) to evaluate underlying physiological responses to hypoxia and determine if hypothalamic CRF and UI are responsible for hypoxia-induced anorexia in channel catfish. During a short exposure to hypoxia (12 h), venous Po2 was significantly lower within 6 h and was coupled with an increase of hematocrit and decrease of blood osmolality, yet all responses reversed within 12 h after returning to normoxia. When this pattern of hypoxia and normoxia was repeated cyclically for 5 days, these physiological responses repeated daily. Extended periods of hypoxia (5 days) resulted in similar hematological responses, which did not recover to baseline values during the hypoxia exposure. This study did not find a significant change in hypothalamic transcription of CRF and UI during hypoxia challenges but did identify multiple physiological adaptive responses that work together to reduce the severity of experimentally induced hypoxia in channel catfish.







Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aboagye DL, Allen PJ (2018) Effects of acute and chronic hypoxia on acid-base regulation, hematology, ion, and osmoregulation of juvenile American paddlefish. J Comp Physiol B 188(1):77–88
Affonso EG, Polez VLP, Correa CF, Mazon AF, Araujo MRR, Moraes G, Rantin FT (2002) Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia. Comp Biochem Physiol C 133:375–382
Arai M, Assil IQ, Abou-Samra AB (2001) Characterization of three corticotropin-releasing factor receptors in catfish: a novel third receptor is predominantly expressed in pituitary and urophysis
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525
Beecham RV, Minchew CD, Parson GR, LaBarre SB (2009) Comparative swimming performance of juvenile blue catfish and hybrid catfish. N Am J Aquac 71:348–353
Bernier NJ (2006) The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen Comp Endocrinol 146:45–55
Bernier NJ, Craig PM (2005) CRF-related peptides contribute to stress response and regulation of appetite in hypoxic rainbow trout. Am J Physiol Regul Integr Comp Physiol 289:R982–R990
Bernier NJ, Peter RE (2001) The hypothalamic–pituitary–interrenal axis and the control of food intake in teleost fish. Comp Biochem Physiol B 129:639–644
Bernier NJ, Alderman SL, Bristow EN (2008) Heads or tails? Stressor-specific expression of corticotropin-releasing factor and urotensin I in the preoptic area and caudal neurosecretory system of rainbow trout. J Endocrinol 196:637–648
Bernier NJ, Gorissen M, Flik G (2012) Differential effects of chronic hypoxia and feed restriction on the expression of leptin and its receptor, food intake regulation and the endocrine stress response in common carp. J Exp Biol 215:2273–2282
Bosworth B, Waldbieser G, Garcia A, Tsuruta S, Lourenco D (2020) Heritability and response to selection for carcass weight and growth in the Delta select strain of channel catfish, Ictalurus punctatus. Aquaculture 515:734507
Boyd CE, Watten BJ, Goubier V, Wu R (1994) Gas supersaturation in surface waters of aquaculture ponds. Aquacult Eng 13:31–39
Boyd CE, Torrans ELT, Tucker CS (2018) Dissolved oxygen and aeration in ictalurid catfish aquaculture. J World Aquac Soc 49(1):7–70
Buentello JA, Gatlin DM III, Neill WH (2000) Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 182:339–352
Burggren WW, Cameron JN (1980) Anaerobic metabolism, gas exchange, and acid-base balance during hypoxic exposure in the channel catfish, Ictalurus punctatus. J Exp Zool 213:405–416
Burleson ML, Smatresk NJ (1990) Effects of sectioning cranial nerves IX and × on cardiovascular and ventilatory reflex responses to hypoxia and NaCN in channel catfish. J Exp Biol 154(1):407–420
Burt K, Hamoutene D, Perez-Casanova J, Gamperl AK, Volkoff H (2014) The effect of intermittent hypoxia on growth, appetite, and some aspects of the immune response of Atlantic salmon (Salmo salar). Aquac Res 45:124–137
Bushnell PG, Steffensen PG, Johansen K (1984) Oxygen consumption and swimming performance in hypoxia-acclimated rainbow trout Salmo gairdneri. J Exp Biol 113:225–235
Chapman LJ, McKenzie DJ (2009) Behavioral responses and ecological consequences. In: Richards JG, Farrell AP, Brauner CJ (eds) Fish physiology, Vol 27: hypoxia. Elsevier, Amsterdam, pp 25–77
Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia: integration through fry’s concept of aerobic metabolic scope. J Fish Biol 88:232–251
Cole BA, Boyd CE (1986) Feeding rate, water quality, and channel catfish production in ponds. Prog Fish-Cult 48(1):25–29
Doyon C, Trudeau VL, Moon TW (2005) Stress elevates corticotropin-releasing factor (CRF) and CRF-binding protein mRNA levels in rainbow trout (Oncorhynchus mykiss). J Endocrinol 186:123–130
Farrell AP (2007) Tribute to P. L. Lutz: a message from the heart–why hypoxic bradycardia in fishes? J Exp Biol 210:1715–1725
Gerald JW, Cech JJ (1970) Respiratory responses of juvenile catfish (Ictalurus punctatus) to hypoxic conditions. Physiol Zool 43(1):47–54
Glass ML, Ishimatsu A, Johansen K (1986) Responses of aerial ventilation to hypoxia and hypercapnia in Channa argus, an air-breathing fish. J Comp Physiol B 156:425–430
Green BW, Rawles SD (2011) Comparative production of channel catfish and channel x blue hybrid catfish subjected to two minimum dissolved oxygen concentrations. N Am J Aquac 73:311–319
Heath AG, Pritchard AW (1965) Effects of severe hypoxia on carbohydrate energy stores and metabolism in two species of fresh-water fish. Physiol Zool 38(4):325–334
Herbert NA, Steffensen JF (2005) The response of Atlantic cod, Gadus morhua, to progressive hypoxia: fish swimming speed and physiological stress. Mar Biol 147:1403–1412
Jensen FB, Fago A, Weber RE (1998) Hemoglobin structure and function. In: Perry SF, Tufts B (eds) Fish physiology, vol 17: fish respiration. Academic Press, San Diego, pp 1–40
Jordan AJ, Steffensen JF (2007) Effects of ration size and hypoxia on specific dynamic action in the cod. Physiol Biochem Zool 80(2):178–185
Lai JCC, Kakuta I, Mok HOL, Rummer JL, Randall D (2006) Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. J Exp Biol 209:2734–2738
Lovejoy DA, Jahan S (2006) Phylogeny of the corticotropin-releasing factor family of peptides in the metazoan. Gen Comp Endocrinol 146:1–8
Magnuson JJ, Beckel AL, Mills K, Brandt SB (1985) Surviving winter hypoxia: behavioral adaptations of fishes in a northern Wisconsin winterkill lake. Envrion Biol Fish 14(4):241–250
Matey V, Richards JG, Wang Y, Wood CM, Rogers J, Davies R, Murray BW, Chen XQ, Du J, Brauner CJ (2008) The effect of hypoxia on gill morphology and ionoregulatory status in the Lake Qinghai scaleless carp, Gymnocypris przewalskii. J Exp Biol 211:1063–1074
Nilsson GE, Östlund-Nilsson S (2008) Does size matter for hypoxia tolerance in fish? Biol Rev 83:173–189
Nilsson GE, Rosen P, Johansson D (1993) Anoxic depression of spontaneous locomotor activity in crucian carp quantified by a computerized imaging technique. J Exp Biol 180:153–162
O’Connor EA, Pottinger TG, Sneddon LU (2011) The effects of acute and chronic hypoxia on cortisol, glucose, and lactate concentrations in different populations of three-spined stickleback. Fish Physiol Biochem 37(3):461–469
Ott BD, Torrans L, Griffin M, Allen PJ (2022b) Quantitative PCR assays to measure the HPI axis neuropeptides corticotropin-releasing factor (CRF) and urotensin I (UI) in channel catfish (Ictalurus punctatus). Aquaculture 555:738253
Ott BD, Torrans EL, Allen PJ (2022) Design of a vacuum degassing apparatus to reduce nitrogen supersaturation and maintain hypoxia in well water. N Am J Aquac 84(4):480–485
Phuong LM, Hyong DTT, Nyengaard JR, Bayley M (2017) Gill remodeling and growth rate of striped catfish (Pangasianodon hypothalamus) under impacts of hypoxia and temperature. Comp Biochem Physiol A 203:288–296
Pichavant K, Person-Le-Ruyet J, Bayon NL, Severe A, Le Roux A, Boeuf G (2001) Comparative effects of long-term hypoxia on growth, feeding and oxygen consumption in juvenile turbot and European sea bass. J Fish Biol 59(4):875–883
Richards JG (2009) Metabolic and molecular responses of fish to hypoxia. In: Richards JG, Farrell AP, Brauner CJ (eds) Fish physiology, Vol 27: hypoxia. Elsevier, Amsterdam, pp 443–485
Schaack S, Chapman LJ (2003) Interdemic variation in the African cyprinid Barbus neumayeri: correlations among hypoxia, morphology, and feeding performance. Can J Zool 81:430–440
Scott AL, Rogers WA (1980) Histological effects of prolonged sublethal hypoxia on channel catfish Ictalurus punctatus (Rafinesque). J Fish Dis 3:305–316
Scott AL, Rogers WA (1981) Haematological effects of prolonged hypoxia on channel catfish* Ictalurus punctatus (Rafinesque). J Fish Biol 18:591–601
Small BC (2003) Anesthetic efficacy of metomidate and comparison of plasma cortisol responses to tricaine methanesulfonate, quinaldine, and clove oil anesthetized channel catfish Ictalurus punctatus. Aquaculture 218(1):177–185
Small BC, Davis KD (2002) Validation of a time-resolved fluoroimmunoassay for measuring plasma cortisol in channel catfish Ictalurus punctatus. J World Aquaculture Soc 33(2):184–187
Small BC, Murdock CA, Bilodeau-Bourgeois AL, Peterson BC, Waldbieser GC (2008) Stability of reference genes for real-time PCR analyses in channel catfish (Ictalurus punctatus) tissues under varying physiological conditions
Sollid J, De Angelis P, Gunderson K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J Exp Biol 206:3667–3673
Steffensen JF, Farrell AP (1998) Swimming performance, venous oxygen tension and cardiac performance of coronary-ligated rainbow trout, Oncorhynchus mykiss, exposed to progressive hypoxia. Comp Biochem Physiol A 119(2):585–592
Steffensen JF, Lomholt JP, Johansen K (1982) Gill ventilation and O2 extraction during graded hypoxia in two ecologically distinct species of flatfish, the flounder (Platichthys flesus) and the plaice (Pleuronectes platessa). Environ Biol Fishes 7(2):157–163
Sundin L, Reid SG, Rantin FT, Milsom WK (2000) Branchial receptors and cardiorespiratory reflexes in a neotropical fish, the Tambaqui (Colossoma macropomum). J Exp Biol 203:1225–1239
Taylor JC, Miller JM (2001) Physiological performance of juvenile southern flounder, Paralichthys lethostigma (Jordan and Gilbert 1984), in chronic and episodic hypoxia. J Exp Mar Biol Ecol 258:195–214
Timmerman CM, Chapman LJ (2004) Behavioral and physiological compensation for chronic hypoxia in the sailfin molly (Poecilia latipinna). Physiol Biochem Zool 77(4):601–610
Tomasso JR, Davis KB, Parker NC (1981) Plasma corticosteroid dynamics in channel catfish, Ictalurus punctatus (Rafinesque), during and after oxygen depletion. J Fish Biol 18:519–526
Torrans EL (2005) Effect of oxygen management on culture performance of channel catfish in earthen ponds. N Am J Aquac 67:275–288
Torrans EL (2008) Production responses of channel catfish to minimum daily dissolved oxygen concentrations in earthen ponds. N Am J Aquac 70:371–381
Torrans L, Ott B, Bosworth B (2015) Impact of minimum daily dissolved oxygen concentration on production performance of hybrid female channel catfish x male blue catfish. N Am J Aquac 77(4):52–56
Torrans L, Ott B, Bosworth B (2012) Impact of minimum dissolved oxygen concentration on grow-out performance of blue catfish comparison to channel catfish. N Am J Aquac 74(2):273–282
Tucker CS (1996) The ecology of channel catfish culture ponds in northwest Mississippi. Rev Fish Sci 4(1):1–55
USDA (United States Department of Agriculture). (2014) Census of aquaculture (2013). USDA, AC-12-SS-2. Beltsville, MD
Val AL (2000) Organic phosphates in the red blood cells of fish. Comp Biochem Physiol A 125:417–435
Val AL, Gomes KRM, de Almeida-Val VMF (2015) Rapid regulation of blood parameters under acute hypoxia in the Amazonian fish Prochilodus nigricans. Comp Biochem Physiol A 184:125–131
Wells RMG, Weber RE (1990) The spleen in hypoxic and exercised rainbow trout. J Exp Biol 150:461–466
Wells RMG, Grigg GC, Beard LA, Summers G (1989) Hypoxic responses in a fish from a stable environment: blood oxygen transport in the Antarctic fish Pagothenia borchgrevniki. J Exp Biol 141:97–111
Wood SC, Johansen K, Weber RE (1975) Effects of ambient Po2 on hemoglobin-oxygen affinity and red cell ATP concentrations in a benthic fish, Pleuronectes platessa. Resp Physiol 25:259–267
Acknowledgements
This project was funded by U. S. Department of Agriculture-Agricultural Research Service project 6402-13320-004-00D, USDA-ARS project 58-6066-5-042, USDA National Institute of Food and Agriculture project 1006942, and the Mississippi Agricultural and Forestry Experiment Station. We would like to thank Landon Chisolm, Reese Mascagni, and Geoff Waldbieser for their assistance in the development and execution of this project. Brian Bosworth and Caitlin Older provided helpful reviews of this manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by USDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Human and animal right statement
All experimental protocols were performed in compliance with the Warmwater Aquaculture Research Unit Institutional Animal Care and Use Committee (IACUC FY17-005).
Additional information
Communicated by G. Heldmaier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ott, B.D., Chisolm, D.O., Griffin, M.J. et al. Effect of hypoxia duration and pattern on channel Catfish (Ictalurus punctatus) neuropeptide gene expression and hematology. J Comp Physiol B 193, 631–645 (2023). https://doi.org/10.1007/s00360-023-01521-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-023-01521-5