Abstract
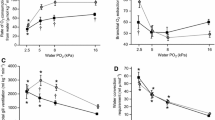
The hagfishes are an ancient and evolutionarily important group, with breathing mechanisms and gills very different from those of other fishes. Hagfish inhale through a single nostril via a velum pump, and exhale through multiple separate gill pouches. We assessed respiratory performance in E. stoutii (31 ppt, 12 ºC, 50–120 g) by measuring total ventilatory flow (\(\dot{\text V}\text{w}\)) at the nostril, velar (respiratory) frequency (fr), and inspired (PIO2) and expired (PEO2) oxygen tensions at all 12 gill pouch exits plus the pharyngo-cutaneous duct (PCD) on the left side, and calculated ventilatory stroke volume (S\(\dot{\text V}\text{w}\)), % O2 utilization, and oxygen consumption (ṀO2). At rest under normoxia, spontaneous changes in \(\dot{\text V}\text{w}\) ranged from apnea to > 400 ml kg−1 min−1, due to variations in both fr and S\(\dot{\text V}\text{w}\); “normal” \(\dot{\text V}\text{w}\) averaged 137 ml kg−1 min−1, ṀO2 was 718 µmol kg−1 h−1, so the ventilatory convection requirement for O2 was about 11 L mmol−1. Relative to anterior gill pouches, lower PEO2 values (i.e. higher utilization) occurred in the more posterior pouches and PCD. Overall, O2 utilization was 34% and did not change during hyperventilation but increased to > 90% during hypoventilation. Environmental hypoxia (PIO2 ~ 8% air saturation, 1.67 kPa, 13 Torr) caused hyperventilation, but neither acute hyperoxia (PIO2 ~ 275% air saturation, 57.6 kPa, 430 Torr) nor hypercapnia (PICO2 ~ 1% CO2, 1.0 kPa, 7.5 Torr) significantly altered \(\dot{\text V}\text{w}\). ṀO2 decreased in hypoxia and increased in hyperoxia but did not change in hypercapnia. Acute exposure to high environmental ammonia (HEA, 10 mM NH4HCO3) caused an acute decrease in \(\dot{\text V}\text{w}\), in contrast to the hyperventilation of long-term HEA exposure described in a previous study. The hypoventilatory response to HEA still occurred during hypoxia and hyperoxia, but was blunted during hypercapnia. Under all treatments, ṀO2 increased with increases in \(\dot{\text V}\text{w}\). Overall, there were lower convection requirements for O2 during hyperoxia, higher requirements during hypoxia and hypercapnia, but unchanged requirements during HEA. We conclude that this “primitive” fish operates a flexible respiratory system with considerable reserve capacity.









Similar content being viewed by others
Abbreviations
- PCO2 :
-
Carbon dioxide tension
- PEO2 :
-
Expired oxygen tension
- HEA:
-
High environmental ammonia
- PIO2 :
-
Inspired oxygen tension
- ṀO2 :
-
Oxygen consumption
- PCD:
-
Pharyngo-cutaneous duct
- fr:
-
Velar (respiratory) frequency
- \(\dot{\text V}\text{w}\) :
-
Ventilatory flow
- S\(\dot{\text V}\text{w}\) :
-
Ventilatory stroke volume
References
Baker DW, Sardella B, Rummer JL, Sackville M, Brauner CJ (2015) Hagfish: champions of CO2 tolerance question the origins of vertebrate gill function. Sci Rep 5:11182
Bardack D (1998) Relationships of living and fossil hagfishes. In: Jorgensen JM, Lomholt JP, Weber RE, Malte H (eds) The biology of Hagfishes. Chapman and Hall, London, pp 3–14
Bartels H (1998) The gills of hagfishes. In: Jorgensen JM, Lomholt JP, Weber RE, Malte H (eds) The biology of Hagfishes. Chapman and Hall, London, pp 205–222
Boutilier RG, Heming TA, Iwama GK (1984) Appendix: physicochemical parameters for use in fish respiratory physiology. In: Hoar WS, Randall DJ (eds) Gills anatomy, gas transfer, and acid-base regulation, fish physiology, vol 10B. Elsevier, Orlando, pp 403–430
Braun CB (1998) Schreiner organs: a new craniate chemosensory modality in hagfishes. J Comp Neurol 392:135–163
Braun CB, Northcutt GR (1998) Cutaneous exteroreceptors and their innervation in hagfishes. In: Jorgensen JM, Lomholt JP, Weber RE, Malte H (eds) The biology of Hagfishes. Chapman & Hall, London, pp 512–532
Braun MH, Perry SF (2010) Ammonia and urea excretion in the Pacific hagfish Eptatretus stoutii: Evidence for the involvement of Rh and UT proteins. Comp Biochem Physiol A 157:405–415
Bushnell PG, Brill RW (1992) Oxygen transport and cardiovascular responses in skipjack tuna (Katsuwonus pelamis) and yellowfin tuna (Thunnus albacares) exposed to acute hypoxia. J Comp Physiol B 162:131–143
Carlson JK, Goldman KJ, Lowe CG (2004) Metabolism, energetic demand, and endothermy. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. NY, CRC Press, New York, pp 203–224
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Clifford AM, Guffey SC, Goss GG (2014) Extra-branchial mechanisms of systemic pH recovery in hagfish (Eptatretus stoutii). Comp Biochem Physiol A Mol Integr Physiol 168:82–89
Clifford AM, Zimmer AM, Wood CM, Goss GG (2016) It’s all in the gills: evaluation of O2 uptake in Pacific hagfish refutes a major respiratory role for the skin. J Exp Biol 219:2814–2818
Clifford AM, Weinrauch AM, Edwards SL, Wilkie MP, Goss GG (2017) Flexible ammonia handling strategies using both cutaneous and branchial epithelia in the highly ammonia-tolerant Pacific hagfish. Am J Physiol Regul Integr Comp Physiol 313:R78–R90
Clifford AM, Weinrauch AM, Goss GG (2018) Dropping the base: recovery from extreme hypercarbia in the CO2 tolerant Pacific hagfish (Eptatretus stoutii). J Comp Physiol B 188:421–435
Coxon SE, Davison W (2011) Structure and function of the velar muscle in the New Zealand hagfish Eptatretus cirrhatus: response to temperature change and hypoxia. J Fish Biol 79:280–289
Davis JC, Cameron JN (1971) Water flow and gas exchange at the gills of rainbow trout, Salmo gairdneri. J Exp Biol 54:1–18
De Boeck G, Wood CM (2015) Does ammonia trigger ventilation in the dogfish shark, Squalus acanthias suckleyi? Respir Physiol Neurobiol 206:25–35
Drazen JC, Yeh J, Friedman J, Condon N (2011) Metabolism and enzyme activities of hagfish from shallow and deep water of the Pacific Ocean. Comp Biochem Physiol A 159:182–187
Eddy FB (1974) Blood gases of the tench (Tinca tinca) in well aerated and oxygen-deficient waters. J Exp Biol 60:71–83
Edwards SL, Arnold J, Blair SD, Pray M, Bradley R, Erikson O, Walsh PJ (2015) Ammonia excretion in the Atlantic hagfish (Myxine glutinosa) and responses of an Rhc glycoprotein. Am J Physiol 308:R769–R778
Eom J, Wood CM (2019) The ventilation mechanism of the Pacific hagfish Eptatretus stoutii. J Fish Biol 94:261–276
Eom J, Giacomin M, Clifford AM, Goss GG, Wood CM (2019) Ventilatory sensitivity to ammonia in the Pacific hagfish (Eptatretus stoutii), a representative of the oldest extant connection to the ancestral vertebrates. J Exp Biol 222, jeb199794. https://doi.org/10.1242/jeb.199794
Eom J, Fehsenfeld S, Wood CM (2020) Is ammonia excretion affected by gill ventilation in the rainbow trout Oncorhynchus mykiss? Respir Physiol Neurobiol 275:103385
Giacomin M, Dal Pont G, Eom J, Schulte PM, Wood CM (2019) The effects of salinity and hypoxia exposure on oxygen consumption, ventilation, diffusive water exchange and ionoregulation in the Pacific hagfish (Eptatretus stoutii). Comp Biochem Physiol A 232:47–59
Giacomin M, Eom J, Schulte PM, Wood CM (2019) Acute temperature effects on metabolic rate, ventilation, diffusive water exchange, osmoregulation and acid-base status in the Pacific hagfish (Eptatretus stoutii). J Comp Physiol B 189:17–35
Gilmour KM (2001) The CO2/pH ventilatory drive in fish. Comp Biochem Physiol A 130:219–240
Goodrich ES (1930) Studies on the structure and development of vertebrates. London, MacMillan and Co. Limited.
Graham MS, Turner JD, Wood CM (1990) Control of ventilation in the hypercapnic skate Raja ocellata. I Blood and extradural fluid. Respir Physiol 80:259–277
Heimberg AM, Cowper-Sal R, Semon M, Donoghue PCJ, Peterson KJ (2010) microRNAs reveal the interrelationship of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Natl Acad Sci USA 107:19379–19383
Heisler N, Toews DP, Holeton GF (1988) Regulation of ventilation and acid-base status in the elasmobranch Scyliorhinus stellaris during hyperoxia-induced hypercapnia. Respir Physiol 71:227–246
Hofbauer M (1934) Anatomischer und histologischer Bau der Kiemensäcke von Myxine glutinosa. Biol General 12:330–348
Holmes WM, Cotton R, Xuan VB, Rygg AD, Craven BA, Abel RL, Slack R, Cox JPL (2011) Three-dimensional structure of the nasal passageway of a hagfish and its implications for olfaction. Anatom Rec 294:1045–1056
Honma Y (1998) Asian hagfishes and their fisheries biology. In: Jorgensen JM, Lomholt JP, Weber RE, Malte H (eds) The Biology of Hagfishes. Champman and Hall, London, pp 45–56
Hughes GM (1960) A comparative study of gill ventilation in marine teleosts. J Exp Biol 52:565–568
Hughes GM (1984) General anatomy of the gills. In: Hoar WS, Randall DJ (eds) Fish Physiology, vol 10A. NY, Academic Press, New York, pp 1–72
Hughes GM, Shelton G (1962) Respiratory mechanisms and their nervous control in fish. In Advances in Comparative Physiology and Biochemistry (ed. O Lowenstein). New York, NY, Academic Press.
Johansen K, Hol RA (1960) A cineradiographic study of respiration in Myxine glutinosa. J Exp Biol 37:474–480
Johansen K, Strahan R (1963) The respiratory system of Myxine glutinosa L. In: Brodal FR (ed) Biology of Myxine. Ostlander, Universitetsforlaget, Oslo, pp 352–371
Jonz MG (2018) Insights into the evolution of polymodal chemoreceptors. Acta Histochem 120:623–629
Lesser MP, Martini FH, Heiser JB (1997) Ecology of the hagfish, Myxine glutinosa L. in the Gulf of Maine I. Metabolic rates and energetics. J Exp Mar Biol Ecol 208:215–225
Lomholt JP, Johansen K (1979) Hypoxia acclimation in carp – how it affects O2 uptake, ventilation and O2 extraction from water. Physiol Zool 52:38–49
Mallatt J (1984) Early vertebrate evolution: pharyngeal structure and the origin of gnathostomes. J Zool Soc Lond 204:169–183
Mallatt J, Paulsen C (1986) Gill ultrastructure of the Pacific hagfish Eptatretus stoutii. Amer J Anatom 177:243–269
Malte H, Lomholt JP (1998) Ventilation and gas exchange. In: Jorgensen JM, Lomholt JP, Weber RE, Malte H (eds) Biology of Hagfishes. Chapman and Hall, London, pp 223–234
Marinelli W, Strenger A (1956) Myxine glutinosa (L.). Vergleichende Anatomie und Morpholgie der Wirbeltiere, Franz Deuticke, Vienna, Bd II:81–172
McInerne JE, Evans DO (1970) Habitat characteristics of the Pacific hagfish, Polistotrema stoutii. J Fish Res Bd Canada 27:966–968
Miyashita T, Coates MI, Farrar R, Larson P, Manning PL, Wogelius RA, Edwards NP, Anne J, Bermann U, Palmer RA, Currie PJ (2019) Hagfish from the cretaceous tethys sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Proc Natl Acad Sci USA 116:2146–2151
Munz FW, Morris R (1965) Metabolic rate of the hagfish, Eptatretus stoutii (Lockington) 1878. Comp Biochem Physiol 16:1–6
Oisi Y, Ota KG, Kuraku S, Fujimoto S, Kuratani S (2013) Craniofacial development of hagfishes and the evolution of vertebrates. Nature 493:175–181
Perry SF, Tzaneva V (2016) The sensing of respiratory gases in fish: mechanisms and signaling pathways. Resp Physiol Neurobiol 224:71–79
Perry SF, Jonz MG, Gilmour KM (2009) Oxygen sensing and the hypoxic ventilatory response. In: Richards JG, Farrell AP, Brauner CJ (eds) Fish physiology. MA, Academic Press, Cambridge, pp 193–252
Perry SF, Vulesevic B, Braun M, Gilmour KM (2009) Ventilation in Pacific hagfish (Eptatretus stoutii) during exposure to acute hypoxia or hypoxia or hypercapnia. Resp Physiol Neurobiol 167:227–234
Piiper J, Schumann DB (1968) Effectiveness of O2 and CO2 exchange in the gills of the dogfish (Scyliorhinus stellaris). Res Physiol 5:338–349
Randall DJ (1970) Gas exchange in fish. In: Hoar WS, Randall DJ (eds) Fish physiology. MA, Academic Press, Cambridge, pp 253–292
Rasmussen AS, Janke A, Arnason U (1998) The mitochondrial DNA molecule of the hagfish (Myxine glutinosa) and vertebrate phylogeny. J Molecul Evol 46:382–388
Smith CR (1985) Food for the deep sea: utilization, dispersal, and flux of nekton falls at the Santa Catalina Basin. Deep Sea Res 32:417–442
Steffensen JF, Johansen K, Sindberg CD, Sørensen JH, Møller JL (1984) Ventilation and oxygen consumption in the hagfish, Myxine glutinosa L. J Exp Mar Biol Ecol 84:173–178
Tambs-Lyche H (1969) Notes on the distribution and ecology of Myxine glutinosa L. Fisk skrifter serie havundersøkelser 15:179–184
Theisen B (1976) The olfactory system in the Pacific hagfishes Eptatretus stoutii, Eptatretus deani, and Myxine circifrons. Acta Zool 57:167–173
Weinrauch AM, Clifford AM, Goss GG (2018) Post-prandial physiology and intestinal morphology of the Pacific hagfish (Eptatretus stoutii). J Comp Physiol B 188:101–112
Wells RM, Forster ME, Davison W, Taylor HH, Davie PS, Satchell GH (1986) Blood oxygen transport in the free-swimming hagfish, Eptatretus cirrhatus. J Exp Biol 43:43–53
Wilkie MP, Clifford A, Edwards SL, Goss GG (2017) Wide scope of ammonia and urea excretion in foraging Pacific hagfish. Mar Biol 164:126
Wood CM, Jackson EB (1980) Blood acid-base regulation during environmental hyperoxia in the rainbow trout (Salmo gairdneri). Respir Physiol 42:351–372
Wood CM, McMahon BR, McDonald DG (1979) Respiratory gas exchange in the resting starry flounder, Platichthys stellatus: a comparison with other teleosts. J Exp Biol 78:167–179
Zhang L, Nurse CA, Jonz MG, Wood CM (2011) Ammonia sensing by neuroepithelial cells and ventilatory responses to ammonia in rainbow trout. J Exp Biol 214:2678–2689
Zhang L, Nawata CM, Wood CM (2013) Sensitivity in ventilation and brain metabolism to ammonia exposure in rainbow trout, Oncorhynchus mykiss. J Exp Biol 216:4025–4037
Acknowledgements
We thank Drs. Bill Milsom, Patricia Schulte, and Tony Farrell for the loan of equipment and advice, Drs. Alex Clifford and Ora Johannsson for advice and access to unpublished data, Ellen Jung and Drs. Beverly Po and Ora Johannsson for help in statistical analysis, and the BMSC Research Co-ordinator, Dr. Eric Clelland for invaluable assistance.
Funding
The research fund was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2017–03843) to CMW.
Author information
Authors and Affiliations
Contributions
JE and CMW conceived the project, JE performed the experiments and generated the data, JE and CMW analyzed the data together, JE wrote the first draft, and CMW edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Ethical approval
The animal usage permits (AUP) were approved by the University of British Columbia (A14-0251, A18-0271) and Bamfield Marine Science Centre (BMSC) animal care committees (AUP RS-17-20, RS-18-20, RS-19-15).
Additional information
Communicated by B. Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eom, J., Wood, C.M. Understanding ventilation and oxygen uptake of Pacific hagfish (Eptatretus stoutii), with particular emphasis on responses to ammonia and interactions with other respiratory gases. J Comp Physiol B 191, 255–271 (2021). https://doi.org/10.1007/s00360-020-01329-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01329-7