Abstract
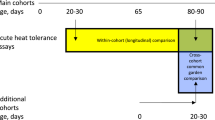
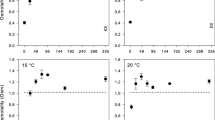
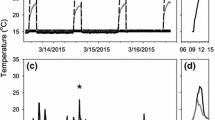
Examples of phenotypic plasticity—the ability of organisms of identical genotypes to produce different phenotypes in response to the environment—are abundant, but often lack data on the causative physiology and biochemistry. Phenotypes associated with increased protection against or reduced damage from harmful environments may, in fact, be downstream effects of hidden adaptive responses that remain elusive to experimental measurement or be obscured by homeostatic or over-compensatory effects. The freshwater zooplankton crustacean Daphnia drastically increases its heat tolerance as the result of acclimation to high temperatures, an effect often assumed to be based on plastic responses allowing better protection against oxidative stress. Using several geographically distant Daphnia magna genotypes, we demonstrate that the more heat tolerant individuals have a higher total antioxidant capacity (TAC) both in the comparison of heat-acclimated vs. non heat-acclimated females and in the comparison of females to age- and body size-matched males, which show lower heat tolerance than females. However, experimental manipulations of hypothesized antioxidant pathways by either glutathione addition or glutathione synthesis inhibition had no effect on heat tolerance. Lipid peroxidation (LPO), contrary to expectations, did not appear to be a predictive measure of susceptibility to thermal damage: LPO was higher, not lower, in more heat tolerant heat-acclimated individuals after exposure to a lethally high temperature. We hypothesize that LPO may be maintained in Daphnia at a constant level in the absence of acute exposure to elevated temperature and increase as a by-product of a possible protective antioxidant mechanism during such exposure. This conclusion is corroborated by the observed short-term and long-term changes in phospholipid composition that included an increase in fatty acid saturation at 28 °C and up-regulation of certain long-chain polyunsaturated fatty acids. Phospholipid composition was more strongly affected by recently experienced temperature (4-day transfer) than by long-term (2 generations) temperature acclimation. This is consistent with partial loss of thermal tolerance after a short-term switch to a reciprocal temperature. As predicted under the homeoviscous adaptation hypothesis, the more heat tolerant Daphnia showed lower membrane fluidity than their less heat tolerant counterparts, in comparison both between acclimation temperatures and among different genotypes. We conclude that thermal tolerance in Daphnia is influenced by total antioxidant capacity and membrane fluidity at high temperatures, with both effects possibly reflecting changes in phospholipid composition.










Similar content being viewed by others
Abbreviations
- BSO:
-
Buthionine sulfoximine
- CumHO:
-
Cumene hydroperoxide
- DPH:
-
1,6-diphenyl-hexa-1,3,5-triene
- DTT:
-
Dithiotreitol
- FDR:
-
False discovery rate
- FI:
-
Fluorescence intensity
- FP:
-
Fluorescence polarization
- GH:
-
Glutathione (reduced)
- LPO:
-
Lipid peroxidation
- TAC:
-
Total antioxidant capacity
- PA:
-
Phosphatidic acids
- PC:
-
Phosphatiditcholines
- PC[n]:
-
Principal component #n
- PCA:
-
Principal component analysis
- PE:
-
Phosphatidylethanolamines
- PFI:
-
Phospholipid fluidity index
- PG:
-
Phosphatidylglycerols
- PI:
-
Phosphatidylinositols
- PS:
-
Phosphatidylserines
- PUFA:
-
Polyunsaturated fatty acid
- ROS:
-
Reactive oxygen species
- SM:
-
Sphingomyelins
- T imm :
-
Time until immobilization
References
Abele D, Burlando B, Viarengo A, Pörtner H (1998) Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpet Nacella concinna. Comp Biochem Physiol Part B 120:425–435
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112:1–14
Bae E, Samanta P, Yoo J, Jung J (2016) Effects of multigenerational exposure to elevated temperature on reproduction, oxidative stress, and Cu toxicity in Daphnia magna. Ecotoxicol Environ Saf 132:366–371
Barata C, Navarro JC, Varo I, Riva MC, Arun S, Porte C (2005a) Changes in antioxidant enzyme activities, fatty acid composition and lipid peroxidation in Daphnia magna during the aging process. Comp Biochem Physiol B Biochem Mol Biol 140:81–90
Barata C, Varo I, Navarro JC, Arun S, Porte C (2005b) Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comp Biochem Physiol C Toxicol Pharmacol 140:175–186
Becker D, Brinkmann B, Zeis B, Paul R (2011) Acute changes in temperature or oxygen availability induce ROS fluctuations in Daphnia magna linked with fluctuations of reduced and oxidized glutathione, catalase. Biol Cell 103:351–363
Bedulina DS, Zimmer M, Timofeyev MA (2010) Sub-littoral and supra-littoral amphipods respond differently to acute thermal stress. Comp. Biochem. Physiol B-Biochem Mol Biol 155:413–418
Beretta G, Aldini G, Facino RM, Russell RM, Krinsky NI, Yeum KJ (2006) Total antioxidant performance: a validated fluorescence assay for the measurement of plasma oxidizability. Anal Biochem 354:290–298
Bhat HA, Kaur T, Bhat R, Vyas D (2016) Physiological and biochemical plasticity of Lepidium latifolium as ‘sleeper weed’ in Western Himalayas. Physiol Plant 156:278–293. doi:10.1111/ppl.12362
Canale CI Henry PY (2010) Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim Res 43:135–147. doi:10.3354/cr00897
Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S. (2013) Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radical Biol Med 65:978–987
de Almeida EA, Bainy ACD, Loureiro APM, Martinez GR, Miyamoto S, Onuki J e al (2009) Oxidative stress in Perna perna and other bivalves as indicators of environmental stress in the Brazilian marine environment: antioxidants, lipid peroxidation and DNA damage. Comp Biochem Physiol A-Mol Integr Physiol 146:588–600. doi:10.1016/j.cbpa.2006.02.040
de Pinto MC, Locato V, Paradiso A, De Gara L (2015) Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann Bot (Lond) 116:487–496
Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA (2002) C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (Micro)Spectroscopic characterization and validation of methodology. Free Radic Biol Med 33:473–490
Fajardo VA, McMeekin L, LeBlanc PJ (2011) Influence of phospholipid species on membrane fluidity: a meta-analysis for a novel phospholipid fluidity index. J Membrane Biol 244:97–103
Farmer EE, Mueller MJ (2013) ROS-Mediated Lipid Peroxidation and RES-Activated Signaling. Annu Rev Plant Biol 64:429–450
Geerts AN, Vanoverbeke J, Vanschoenwinkel B, Van Doorslaer W, Feuchtmayr H, Atkinson D, Moss B, Davidson TA, Sayer CD, De Meester L (2015) Rapid evolution of thermal tolerance in the water flea Daphnia. Nat Clim Change 5:665–668
Giordano E, Visioli F (2013) Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fatty Acids (PLEFA) 90:1–4. doi:10.1016/j.plefa.2013.11.002
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947. doi:10.1038/nrd4002
Gueraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K (2010) Chemistry and biochemistry of lipid peroxidation products. Free Radical Res 44:1098–1124. doi:10.3109/10715762.2010.498477
Halliwell B (2000) Lipid peroxidation, antioxidants and cardiovascular disease: how should we move forward? Cardiovasc Res 47:410–418
Hazel JR, Williams E (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–277. doi:10.1016/0163-7827(90)90002-3
Hoffmann AA, Willi Y (2008) Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432
Jolliffe IT (1986) Principal component analysis. Springer-Verlag. p. 487
Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L (1998) COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159
Klumpen E, Hoffschröer N, Zeis B, Gigengack U, Dohmen E, Paul RJ (2016) Reactive oxygen species (ROS) and the heat stress response of Daphnia pulex: ROS-mediated activation of hypoxia-inducible factor 1 (HIF-1) and heat shock factor 1 (HSF-1) and the clustered expression of stress genes. Biol Cell. In press, 2016, Aug 12. doi:10.1111/boc.201600017. (Epub ahead of print)
Kushnareva Y (2009) Membrane Fluidity Measurements Using UV Fluorescence Polarization and the POLARstar Omega. LabTech Application note. Available: http://www.bmglabtech.com/media/35216/1043850.pdf
Libralato G, Prato E, Migliore L, Cicero AM, Manfra L (2016) A review of toxicity testing protocols and endpoints with Artemia spp. Ecol Indic 69:35–49. doi:10.1016/j.ecolind.2016.04.017
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Martin-Creuzburg D, Wacker A, Ziese C, Kainz MJ (2012) Dietary lipid quality affects temperature-mediated reaction norms of a freshwater key herbivore. Oecologia 168:901–912. doi:10.1007/s00442-011-2155-1
Maulucci G, Daniel B, Cohen O, Avrahami Y, Sasson S (2016) Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol Aspects Med 49:49–77
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92
Morris M, Rogers SM. (2014) Integrating phenotypic plasticity within an ecological genomics framework: Recent insights from the genomics, evolution, ecology, and fitness of plasticity. In: Landry CR, Aubin-Horth N, eds. Ecological Genomics: Ecology And The Evolution Of Genes And Genomes. Advances In Experimental Medicine And Biology 781:73–105
Mylonas C, Kouretas D (1999) Lipid peroxidation and tissue damage. In Vivo 13:295–309
Oexle S, Jansen M, Pauwels K, Sommaruga R, De Meester L, Stoks R (2016) Rapid evolution of antioxidant defence in a natural population of Daphnia magna. J Evol Biol 29:1328–1337. doi:10.1111/jeb.12873
Oomen RA, Hutchings JA (2015) Genetic variability in reaction norms in fishes. Environ Rev 23:353–366. doi:10.1139/Z86-007 10.1139/er-2014-0077
Pap EH, Drummen GP, Winter VJ, Kooij TW, Rijken P, Wirtz K.W, Op den Kamp JA, Hage WJ, Post JA (1999) Ratio-fluorescence microscopy of lipid oxidation in living cells using C11-BODIPY(581/591). Febs Lett 453:278–282
Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP (2010) Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25:459–467. doi:10.1016/j.tree.2010.05.006
Podosinovikova NP, Petrov VV, Dolgo-Saburov VB (2005) Daphnia magna Straus: a new model for evaluating the antioxidant action of water-soluble preparations in vivo. Exper Clin Pharmacol 68:68–70 (Russian)
Richard D, Kefi K, Barbe U, Bausero P, Visioli F (2008) Polyunsaturated fatty acids as antioxidants. Pharmacol Res 57:451–455. doi:10.1016/j.phrs.2008.05.002
Rodriguez-Martinez MA, Ruiz-Torres A (1992) Homeostasis between lipid peroxidation and antioxidant enzyme activities in healthy human aging. Mech Ageing Dev 66:213–222
Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW (1984) Hemoglobin. A biologic fenton reagent. J Biol Chem 259:14354–14356
SAS Institute (2012) JMP Statistical software. SAS Institute, Inc, Cary
Schlechtriem C, Arts MT, Zellmer ID (2006) Effect of temperature on the fatty acid composition and temporal trajectories of fatty acids in fasting Daphnia pulex (Crustacea, Cladocera). Lipids 41:397–400
Sgro CM, Terblanche JS, Hoffmann AA (2016) What can plasticity contribute to insect responses to climate change? Annu Rev Entomol 61:433–451. doi:10.1146/annurev-ento-010715-023859
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
van Kleunen M, Fischer M (2005) Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol 166:49–60
Williams PJ, Dick KB, Yampolsky LY (2011) Heat tolerance, temperature acclimation, acute oxidative damage and canalization of haemoglobin expression in Daphnia. Evol Ecol 26:591–609
Yampolsky LY, Glazko GV, Fry JD (2012) Evolution of gene expression and expression plasticity in long-term experimental populations of maintained under constant and variable ethanol stress. Mol Ecol 21(17):4287–4299
Yampolsky LY, Schaer TMM, Ebert D (2014) Adaptive phenotypic plasticity and local adaptation for temperature tolerance in freshwater zooplankton. Proc R Soc Ser B Biol Sci 281:20132744
Yoshida Y, Shimakawa S, Itoh N, Niki E (2003) Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain. Free Radic Res 8:861–872
Zeis B, Lamkemeyer T, Paul RJ, Nunes F, Schwerin S, Koch M, Schütz W, Madlung J, Fladerer C, Pirow R (2009) Acclimatory responses of the Daphnia pulex proteome to environmental changes. I. Chronic exposure to hypoxia affects the oxygen transport system and carbohydrate metabolism. BMC Physiol 9:7
Zeis B, Becker D, Gerke P, Koch M, Paul RJ (2013) Hypoxia-inducible haemoglobins of Daphnia pulex and their role in the response to acute and chronic temperature increase. Biochim Biophys Acta 1834:1704–1710.
Zhou KI, Pincus Z, Slack FJ (2011) Longevity and stress in Caenorhabditis elegans. Aging-US 3:733–753
Zinellu E, Zinellu A, Fois AG, Carru C, Pirina P (2016) Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: a systematic review. Respir Res 17:150. doi:10.1186/s12931-016-0471-z
Acknowledgements
We are grateful to Dhirendra Kumar for equipment use, to Anthony C. Tharp and Irina Kaverina for assistance with fluorescence polarization measurements and to Joe Bidwell, Dieter Ebert, Karl Joplin, Aruna Kilaru, Dominik Martin-Creuzburg, and Roberto Arbore for many useful suggestions. This work was partly supported by NSF DEB-1136706 to LYY and ETSU Student-Faculty Collaboration grants to BLC and JWC.
Author contributions
LYY designed the study, participated in the experimental work, analyzed the data, and wrote the manuscript. BLC, JC, and KJH did the experimental work and participated in data analysis and writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
B. L. Coggins, J. W. Collins, and K. J. Holbrook contributed equally to the work and are listed alphabetically.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coggins, B.L., Collins, J.W., Holbrook, K.J. et al. Antioxidant capacity, lipid peroxidation, and lipid composition changes during long-term and short-term thermal acclimation in Daphnia . J Comp Physiol B 187, 1091–1106 (2017). https://doi.org/10.1007/s00360-017-1090-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1090-9