Abstract
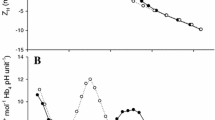
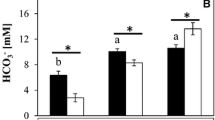
The plainfin midshipman (Porichthys notatus) possesses an aglomerular kidney and like other marine teleosts, secretes base into the intestine to aid water absorption. Each of these features could potentially influence acid–base regulation during respiratory acidosis either by facilitating or constraining HCO3 − accumulation, respectively. Thus, in the present study, we evaluated the capacity of P. notatus to regulate blood acid–base status during exposure to increasing levels of hypercapnia (nominally 1–5% CO2). Fish exhibited a well-developed ability to increase plasma HCO3 − levels with values of 39.8 ± 2.8 mmol l−1 being achieved at the most severe stage of hypercapnic exposure (arterial blood PCO2 = 21.9 ± 1.7 mmHg). Consequently, blood pH, while lowered by 0.15 units (pH = 7.63 ± 0.06) during the final step of hypercapnia, was regulated far above values predicted by chemical buffering (predicted pH = 7.0). The accumulation of plasma HCO3 − during hypercapnia was associated with marked increases in branchial net acid excretion (J NETH+) owing exclusively to increases in the titratable alkalinity component; total ammonia excretion was actually reduced during hypercapnia. The increase in J NETH+ was accompanied by increases in branchial carbonic anhydrase (CA) enzymatic activity (2.8×) and CA protein levels (1.6×); branchial Na+/K+-ATPase activity was unaffected. Rectal fluids sampled from control fish contained on average HCO3 − concentrations of 92.2 ± 4.8 mmol l−1. At the highest level of hypercapnia, rectal fluid HCO3 − levels were increased significantly to 141.8 ± 7.4 mmol l−1 but returned to control levels during post-hypercapnia recovery (96.0 ± 13.2 mmol l−1). Thus, the impressive accumulation of plasma HCO3 − to compensate for hypercapnic acidosis occurred against a backdrop of increasing intestinal HCO3 − excretion. Based on in vitro measurements of intestinal base secretion in Ussing chambers, it would appear that P. notatus did not respond by minimizing base loss during hypercapnia; the increases in base flux across the intestinal epithelium in response to alterations in serosal HCO3 − concentration were similar in preparations obtained from control or hypercapnic fish. Fish returned to normocapnia developed profound metabolic alkalosis owing to unusually slow clearance of the accumulated plasma HCO3 −. The apparent inability of P. notatus to effectively excrete HCO3 − following hypercapnia may reflect its aglomerular (i.e., non-filtering) kidney coupled with the normally low rates of urine production in marine teleosts.






Similar content being viewed by others
References
Baker DW, Matey V, Huynh KT, Wilson JM, Morgan JD, Brauner CJ (2009) Complete intracellular pH protection during extracellular pH depression is associated with hypercarbia tolerance in white sturgeon, Acipenser transmontanus. Am J Physiol 296:R1868–R1880
Baustian MD, Wang SQ, Beyenbach KW (1997) Adaptive responses of aglomerular toadfish to dilute sea water. J Comp Physiol B 167:61–70
Beyenbach KW (2004) Kidneys sans glomeruli. Am J Physiol 286:F811–F827
Boutilier RG, Heming TA, Iwama GK (1984) Physiochemical parameters for use in fish respiratory physiology. In: Hoar WS, Randall DJ (eds) Fish physiology, vol XA. Academic Press, New York, pp 403–430
Brauner CJ, Wang T, Wang Y, Richards JG, Gonzalez RJ, Bernier NJ, Xi W, Patrick A, Va AL (2004) Limited extracellular but complete intracellular acid–base regulation during short-term environmental hypercapnia in the armoured catfish, Liposarcus pardalis. J Exp Biol 207:3381–3390
Cameron JN (1976) Branchial ion uptake in Arctic grayling: resting values and the effects of acid–base disturbance. J Exp Biol 64:711–725
Cameron JN (1978) Regulation of blood pH in teleost fish. Respir Physiol 33:129–144
Cameron JN, Iwama GK (1989) Compromises between ionic regulation and acid–base regulation in aquatic animals. Can J Zool 67:3078–3084
Cameron JN, Randall DJ (1972) The effect of increased ambient CO2 on arterial CO2 tension, CO2 content and pH in rainbow trout. J Exp Biol 57:673–680
Claiborne JB, Compton-McCullough D, Walton JS (2000) Branchial acid–base transfers in the euryhaline oyster toadfish during exposure to dilute sea water. J Fish Biol 56:1539–1544
Claiborne JB, Edwards SL, Morrison-Shetlar AI (2002) Acid–base regulation in fishes: cellular and molecular mechanisms. J Exp Zool 293:302–319
Cross CE, Packer BS, Linta JM, Murdaugh H V Jr, Robin ED (1969) H+ buffering and excretion in response to acute hypercapnia in the dogfish Squalus acanthias. Am J Physiol 216:440–452
Curtis BJ, Wood CM (1991) The function of the urinary bladder in vivo in the freshwater rainbow trout. J Exp Biol 155:567–583
Deigweiher K, Koschnick N, Portner HO, Lucassen M (2008) Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol 295:R1660–R1670
Dimberg K (1988) High blood CO2 levels in rainbow trout exposed to hypercapnia in bicarbonate-rich hard fresh water—a methodological verification. J Exp Biol 134:463–466
Evans DH (1982) Mechanisms of acid extrusion by two marine fishes; the teleost, Opsanus beta, and the elasmobranch, Squalus acanthias. J Exp Zool 97:289–299
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Genz J, Taylor JR, Grosell M (2008) Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid–base balance in Opsanus beta. J Exp Biol 211:2327–2335
Georgalis T, Perry SF, Gilmour KM (2006a) The role of branchial carbonic anhydrase in acid–base regulation in rainbow trout (Oncorhynchus mykiss). J Exp Biol 209:518–530
Georgalis T, Yorston J, Gilmour KM, Perry SF (2006b) The roles of cytosolic and membrane bound carbonic anhydrase in the renal control of acid–base balance in rainbow trout, Oncorhynchus mykiss. Am J Physiol 291:F407–F421
Gilmour KM, Perry SF (2009) Carbonic anhydrase and acid–base regulation in fish. J Exp Biol 212:1647–1661
Goss GG, Perry SF (1994) Different mechanisms of acid–base regulation in rainbow trout (Oncorhynchus mykiss) and American eel (Anguilla rostrata) during NaHCO3 infusion. Physiol. Zool 67:381–406
Goss GG, Perry SF, Wood CM, Laurent P (1992) Mechanisms of ion and acid–base regulation at the gills of freshwater fish. J Exp Zool 263:143–159
Grosell M (2006) Intestinal anion exchange in marine fish osmoregulation. J Exp Biol 209:2813–2827
Grosell M, Genz J (2006) Ouabain-sensitive bicarbonate secretion and acid absorption by the marine teleost fish intestine play a role in osmoregulation. Am J Physiol 291:R1145–R1156
Grosell M, Wood CM, Wilson RW, Bury NR, Hogstrand C, Rankin C, Jensen FB (2005) Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am J Physiol 288:R936–R946
Grosell M, Genz J, Taylor JR, Perry SF, Gilmour KM (2009) Secretion of HCO3 − by pyloric caeca and anterior intestine of seawater acclimated rainbow trout: Involvement of apical H+-VATPase and carbonic anhydrase. J Exp Biol 212:1940–1948
Haswell MS, Randall DJ, Perry SF (1980) Fish gill carbonic anhydrase: acid–base regulation or salt transport? Am J Physiol 238:240–245
Heisler N (1978) Bicarbonate exchange between body compartments after changes of temperature in the larger spotted dogfish (Scyliorhinus stellaris). Respir Physiol 33:145–160
Heisler N (1984) Acid–base regulation in fishes. In: Hoar WS, Randall DJ (eds) Fish physiology, vol XA. Academic Press, New York, pp 315–401
Heisler N (1989) Interactions between gas exchange, metabolism, and ion transport in animals: an overview. Can J Zool 67:2923–2935
Henry RP (1991) Techniques for measuring carbonic anhydrase activities in vitro: the electrometric delta pH and pH stat assays. In: Dodgson SJ, Tashian RE, Gros G, Carter ND (eds) The carbonic anhydrases: cellular physiology and molecular genetics. Plenum, New York, pp 119–126
Henry RP, Swenson ER (2000) The distribution and physiological significance of carbonic anhydrase in vertebrate gas exchange organs. Respir Physiol 121:1–12
Hickman CP, Trump BF (1969) The kidney. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York, pp 91–239
Hills AG (1973) Acid–base balance: chemistry, physiology, pathophysiology. Williams and Wilkins, Baltimore
Iwama GK, Heisler N (1991) Effect of environmental water salinity on acid–base regulation during environmental hypercapnia in the rainbow trout (Oncorhynchus mykiss). J Exp Biol 158:1–18
Janssen RG, Randall DJ (1975) The effects of changes in pH and PCO2 in blood and water on breathing in rainbow trout, Salmo gairdneri. Respir Physiol 25:235–245
Larsen BK, Jensen FB (1997) Influence of ionic composition on acid–base regulation in rainbow trout (Oncorhynchus mykiss) exposed to environmental hypercapnia. Fish Physiol Biochem 16:157–170
Lloyd R, White WR (1967) Effect of high concentration of carbon dioxide on the ionic composition of rainbow trout blood. Nature 216:1341–1342
Marshall WS, Grosell M (2006) Ion transport, osmoregulation and acid–base balance. In: Evans DH, Claiborne JB (eds) The physiology of fishes. CRC Press, Boca Raton, pp 177–230
McCormick SD (1993) Methods for non-lethal gill biopsy and measurements of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50:656–658
McDonald MD, Walsh PJ (2007) Aglomerular kidney function when challenged with exogenous MgSO4 loading or environmental MgSO4 depletion. J. Exp. Zool A 307A:676–687
McDonald DG, Wood CM (1981) Branchial and renal acid and ion fluxes in the rainbow trout, Salmo gairdneri, at low environmental pH. J Exp Biol 93:101–118
McDonald DG, Tang Y, Boutilier RG (1989) Acid and ion transfer across the gills of fish: mechanisms and regulation. Can J Zool 67:3046–3054
McDonald MD, Walsh PJ, Wood CM (2002) Branchial and renal excretion of urea and urea analogues in the plainfin midshipman, Porichthys notatus. J Comp Physiol B 172:699–712
Mckenzie DJ, Piccolella M, Dalla Valle AZ, Taylor EW, Bolis CL, Steffensen JF (2003) Tolerance of chronic hypercapnia by the European eel Anguilla anguilla. J Exp Biol 206:1717–1726
Perry SF (1981) The regulation of hypercapnic acidosis in two salmonids, the freshwater trout (Salmo gairdneri) and the seawater salmon (Onchorynchus kisutch). Mar. Behav. Physiol 9:73–79
Perry SF, Gilmour KM (2006) Acid–base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir. Physiol. Neurobiol 154:199–215
Perry SF, Laurent P (1990) The role of carbonic anhydrase in carbon dioxide excretion, acid–base balance and ionic regulation in aquatic gill breathers. In: Truchot JP, Lahlou B (eds) Transport, respiration and excretion: comparative and environmental aspects. Karger, Basel, vol 6, pp 39–57
Perry SF, Haswell MS, Randall DJ, Farrell AP (1981) Branchial ionic uptake and acid–base regulation in the rainbow trout, Salmo gairdneri. J Exp Biol 92:289–303
Perry SF, Malone S, Ewing D (1987a) Hypercapnic acidosis in rainbow trout (Salmo gairdneri). II Renal ionic fluxes. Can J Zool 65:896–902
Perry SF, Malone S, Ewing D (1987b) Hypercapnic acidosis in the rainbow trout (Salmo gairdneri). I. Branchial ionic fluxes and blood acid–base status. Can J Zool 65:888–895
Perry SF, Furimsky M, Bayaa M, Georgalis T, Nickerson JG, Moon TW (2003a) Integrated involvement of Na+/HCO3 − cotransporters and V-type H+-ATPases in branchial and renal acid–base regulation in freshwater fishes. Biochem. Biophys. Acta 1618:175–184
Perry SF, Shahsavarani A, Georgalis T, Bayaa M, Furimsky M, Thomas SLY (2003b) Channels, pumps and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid–base regulation. J Exp Zool 300:53–62
Qin Z, Lewis J, Perry SF (2010) Zebrafish (Danio rerio) gill neuroepithelial cells are sensitive chemoreceptors for environmental CO2. J. Physiol. (Lond.) 588:61–872
Randall DJ, Heisler N, Drees F (1976) Ventilatory response to hypercapnia in the larger spotted dogfish Scyliorhinus stellaris. Am J Physiol 230(3):590–594
Randall DJ, Perry SF, Heming TA (1982) Gas transfer and acid–base regulation in salmonids. Comp. Biochem. Physiol. B 73:93–103
Taylor JR, Mager EM, Grosell M (2010) Basolateral NBCe1 plays a rate-limiting role in transepithelial intestinal HCO3 − secretion, contributing to marine fish osmoregulation. J Exp Biol 213:459–468
Toews DP, Holeton GF, Heisler N (1983) Regulation of the acid–base status during environmental hypercapnia in the marine teleost fish Conger conger. J Exp Biol 107:9–20
Tohse H, Ando H, Mugiya Y (2004) Biochemical properties and immunohistochemical localization of carbonic anhydrase in the sacculus of the inner ear in the salmon Oncorhynchus masou. Comp. Biochem. Physiol. A 137:87–94
Verdouw H, van Echteld CJA, Dekkers EMJ (1978) Ammonia determinations based on indophenol formation with sodium salicylate. Water Res 12:399–402
Wheatly MG, Hobe H, Wood CM (1984) The mechanisms of acid–base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia. II The role of the kidney. Respir Physiol 55:155–173
Wilson RW, Gilmour KM, Henry RP, Wood CM (1996) Intestinal base excretion in the seawater-adapted rainbow trout: a role in acid–base balance? J Exp Biol 199:2331–2343
Wilson RW, Millero FJ, Taylor JR, Walsh PJ, Christensen V, Jennings S, Grosell M (2009) Contribution of fish to the marine inorganic carbon cycle. Science 323:359–362
Wood CM (1991) Branchial ion and acid-base transfer in freshwater teleost fish—environmental hyperoxia as a probe. Physiol. Zool 64:68–102
Wood CM, Jackson EB (1980) Blood acid–base regulation during environmental hyperoxia in the rainbow trout (Salmo gairdneri). Respir Physiol 42:351–372
Wood CM, Wheatly MG, Hobe H (1984) The mechanisms of acid–base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia. III. Branchial exchanges. Respir Physiol 55:175–192
Wood CM, Gilmour KM, Perry SF, Part P, Laurent P, Walsh PJ (1998) Pulsatile urea excretion in gulf toadfish (Opsanus beta): evidence for activation of a specific facilitated diffusion transport system. J Exp Biol 201:805–817
Acknowledgments
The costs of research were supported by Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery and Research Tools and Innovation grants to S.F.P. and K.M.G. and an NSF grant (IAB 0743903) to M.G. M.H.B., B.V. and J.T. received Journal of Experimental Biology Travelling Fellowships. We are grateful to local fish providers (Robert, Delores and James Bowker of Bamfield, BC and Raymond Martel of Parksville, BC). We are forever indebted to Dr. Bruce Cameron, BMSC Research Director, for his tireless support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Perry, S.F., Braun, M.H., Genz, J. et al. Acid–base regulation in the plainfin midshipman (Porichthys notatus): an aglomerular marine teleost. J Comp Physiol B 180, 1213–1225 (2010). https://doi.org/10.1007/s00360-010-0492-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0492-8