Abstract
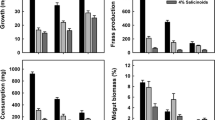
Effects of dietary nicotine and macronutrient ratio on M. sexta larvae were examined. Larvae were fed a carbohydrate-biased, protein-biased or diet having equal amounts of casein and sucrose, with and without nicotine. Without nicotine, larvae displayed compensatory feeding on the low protein diet, but despite consuming more, grew least on this diet. Nicotine at 0.5% had no effect on nutrient consumption. Nicotine at 1.0 and 2.0% reduced overall consumption and thereby also reduced nicotine consumption. Larvae parasitized by C congregata displayed reduced nutrient intake and growth on all diets. Parasitized larvae responded to 1% nicotine similarly to unparasitized larvae. At 0.5% nicotine, they displayed reduced consumption on all diets, possibly due to altered chemoreceptor sensitivity to nicotine. When offered a choice of two diets having different macronutrient ratios, one with and the other without 0.1% nicotine, all larvae preferred the diet lacking nicotine and failed to regulate nutrient intake such that the nutrient intake target, a ratio of nutrients supporting optimal growth, was achieved. Parasitized larvae consumed less nicotine on a fresh weight basis than unparasitized insects, suggesting that the feeding response of parasitized larvae to nicotine minimizes the exposure of nicotine to developing parasites.






Similar content being viewed by others
References
Ahmad IM, Waldbauer GP, Friedman S (1989) A defined artificial diet for the larvae of Manduca sexta. Entomol Exp Appl 53:189–192
Akehurst BC (1981) Tobacco. Longman, New York, pp 522–577
Alleyne M (1995) Alterations in host growth and metabolism of Manduca sexta larvae parasitized by Cotesia congregata, MS Thesis. University of California, Riverside
Appel HM, Martin MM (1992) Significance of metabolic load in the evolution of host specificity in Manduca sexta. Ecology 73:216–228
Bacon CW, Wenger R, Bullock JF (1952) Chemical changes in tobacco during flue-curing. Ind Engng Chem Ind Edg 44:292–296
Baker FC, Tsai LW, Reuter CC, Schooley DA (1987) In vivo fluctuations of JH, JH acid, and ecdysteroid titer, and JH esterase activity, during development of fifth stadium Manduca sexta. Insect Biochem 17:989–996
Bell RA, Joachim RG (1976) Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am 69:365–373
Bernays EA (1998) Evolution of feeding behavior in insect herbivores. BioScience 48:35–44
Bernays EA (1999) Plasticity and the problem of choice in food selection. Ann Entomol Soc Am 92:944–951
Bernays EA, Chapman RF (1987) Evolution of deterrent responses in plant-feeding insects. In: Chapman FR, Bernays EA, Stoffolano JG (eds) Perspectives in chemoreception and behavior. Springer, New York, pp 1–16
Bernays EA, Chapman RF (1994) Host plant selection by phytophagous. Chapman and Hall, New York
Bernays EA, Singer MS (2005) Insect defenses: taste alteration and endoparasites. Nature Lond 436:476
Bezzerides A, Yong T-H, Bezzerides J, Husseini J, Ladau J, Eisner M, Eisner T (2004) Plant-derived pyrrolizidine alkaloid protects eggs of a moth (Utetheisa ornatrix) against a parasitoid wasp (Trichogramm ostriniae). Proc Nat Acad Sci USA:9029–9032
Bowman DR, Nichols BC, Jeffrey RN (1958) Time of harvest–its effect on the chemical composition of individual leaves of burley tobacco. Tenn Agric Exp Sta Bull 291
Brodeur J, Boivin G (2004) Functional ecology of immature parasitoids. Annu Rev Entomol. 49:27–49
Burton HR, Dye NK, Bush LP (1992) Distribution of tobacco constituents in tobacco leaf tissue. 1. tobacco-specific nitrosamines, nitrate, nitrite and alkaloids. J Agric Food Chem 40:1050–1055
Bush LP (1999) Alkaloid biosynthesis. In: Davis DL, Nielsen MT (eds) Tobacco – production, chemistry and technology. Blackwell Scientific, Oxford, pp 285–291
Duffy SS, Bloem KA, Campbell BC (1986) Consequences of sequestration of plant natural products in plant-insect-parasitioid interactions. In: Boethel DJ, Eikenbary RD (eds) Interactions of plant resistance and parasitoids and predators of insects. Elis Horwood, Chichester, pp 31–59
Feeny P (1992) The evolution of chemical ecology: contributions from the study of herbivorous insects. In: Rosenthal GA, Berenbaum MR (eds) Herbivores. their interactions with secondary plant metabolites. Academic Press, New York, pp 1–44
Futuyma DJ, Keese MC (1992) Evolution and coevolution of plants and phytophagous arthropods. In: Rosenthal GA, Berenbaum MR (eds) Herbivores. their interactions with secondary plant metabolites. Academic Press, New York pp 440–475
Glendinning JI (1996) Is chemosensory input essential for the rapid rejection of toxic foods? J Exp Biol 199:1523–1534
Glendinning JI (2002) How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomol Exp Appl 104:15–25
Glendinning JI, Slansky F (1994) Interactions of allelochemicals with dietary constituents: effects on deterrency. Physiol Entomol 19:173–186
Hartman T, Theuring C, Beuerle T, Ernst L, Singer MS, Bernays EA (2004) Acquired and partially de novo synthesized pyrrolizidine alkaloids in two polyphagous arctiids and the alkaloid profiles of their larval food-plants. J Chem Ecol 30:229–254
Horton DR, Redak RA (1993) Further comments on analylsis of covariance in insect dietary studies. Entomol Exp Appl 69:263–275
Jeffrey RN (1958) Buildup and conversion of alkaloids within the tobacco leaf. Cigar Manuf Assn Res Session, Washington, DC
Jeffrey RN (1959) Alkaloid composition of species of Nicotiana. Tob Sci 3:89–93
Kester KM, Peterson SC, Hanson F, Jackson DM, Severson RF (2002) The roles of nicotine and natural enemies in determining larval feeding site distributions of Manduca sexta L. and Manduca quinquemaculata (Haworth) on tobacco. Chemoecology 12:1–10
Leffingwell JC (1999) Leaf chemistry. Basic chemical constituents of tobacco leaf and differences among tobacco types. In: Davis DL, Nielsen MT (eds) Tobacco production, chemistry and technology. Blackwell, London, pp 265–284
McFadden MW (1968) Observations on feeding and movement of tobacco hornworm larvae. J Econ Entomol 61:352–356
Murray CL, Quaglia M, Arnason JT, Morris CE (1994) A putative nicotine pump at the metabolic blood-brain barrier of the tobacco hornworm. J Neurobiol 25:23–34
Parr JC, Thurston R (1972) Toxicity of nicotine in synthetic diets to larvae of the tobacco hornworm. Ann Entomol Soc Am 65:1185–1188
Raubenheimer D, Simpson SJ (1990) The effect of simultaneous variation in protein, digestible carbohydrate and tannic acid on the feeding behaviour of larval Locusta migratoria (L.) and Schistocerca gregaria (Forskal). I. Short-term studies. Physiol Entomol 15:219–233
Raubenheimer D, Simpson SJ (1992) Analysis of covariance: an alternative to nutritional indices. Entomol Exp Appl 62:221–231
Raubenheimer D, Simpson SJ (1997) Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev 10:151–179
Raubenheimer D, Simpson SJ (1999) Integrating nutrition: a geometrical approach. Entomol Exp Appl 91:67–82
Raubenheimer D, Simpson SJ (2003) Nutrient balancing in grasshoppers: behavioural and pyhysiological correlates of dietary breadth. J Exp Biol 206:1669–1681
Reynolds SE, Yeomans MR, Timmins WA (1986) The feeding behaviour of caterpillars (Manduca sexta) on tobacco and on artificial diet. Physiol Entomol 11:39–51
Saitoh F, Noma M, Kawashima N (1985) The alkaloid contents of sixty Nicotiana species. Phytochemistry 24:477–480
Schoonhoven LM (1973) Plant recognition by lepidopterous larvae In: van Emden HF (ed) R.E.S. Symposium 6. Insect/Plant Relationships. Blackwell Scientific, Oxford, pp 87–99
Schoonhoven LM, Blaney WM, Simmonds MSJ (1992) Sensory coding of feeding deterrenbts in phytophagous insects. In: Bernays E (ed) Insect-Plant Interactions Vol. 5, CRC Press, Boca Raton, pp 59–79
Schoonhoven LM, Jermy T, van Loon JJA (1998) Insect-plant biology: from physiology to evolution. Chapman and Hall, New York
Simpson SJ, Raubenheimer D (1993) The central role of haemolymph in the regulation of nutrient intake in insects. Physiol Entomol 18:395–403
Simpson SJ, Raubenheimer D (2001) The geometric analysis of nutrient-allelochemical interactions: a case study using locusts. Ecology 82:422–439
Singer MS, Stiremann III JO (2003) Does anti-parasitoid defense explain host-plant selection by a polyphagous caterpillar? Oikos 100:554–562
Singer MS, Carriere Y, Theuring C, Hartmann T (2004) Disentangling food quality from resistance against parasitoids: diet choice by a generalist caterpillar. Amer Nat 164:423–429
Sisson VA, Saunders JA (1982) Alkaloid composition of the USDA tobacco (Nicotiana tabacum L.) introduction collection. Tobacco Sci 26:117–120
Sisson VA, Saunders JA (1983) Catalog of the tobacco introductions in the US Department of Agriculture’s tobacco germplasm collections (Nicotiana tabacum). Supplement I. Alkaloid content of the cured leaf. USDA., AR. Agric Rev Manuals 27:1–27
Slansky F (1992) Allelochemical-nutrient interactions in herbivore nutritional ecology. In: Rosenthal GA, Berenbaum MR (eds) Herbivores. their interactions with secondary plant metabolites. Academic Press, New York, pp 135–174
Slansky F, Wheeler GS (1992) Caterpillars’ compensatory feeding response to diluted nutrients leads to toxic allelochemical dose. Entomol Exp Appl 65:171–186
Snyder MS, Glendinning JI (1996) Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. J Comp Physiol A 179:255–261
Snyder MJ, Walding JK, Feyereisen R (1994) Metabolic fate of the allelochemical nicotine in the tobacco hornworm Manduca sexta. Insect Biochem Molec Biol 24:837–846
Southwood TRE (1973) The insect/plant relationship–an evolutionary perspective. In: van Emden HF (ed) R.E.S. Symposium 6. Insect/Plant Relationships. Blackwell Scientific, Oxford, pp 3–30
Stadler E (1992) Behavioral responses of insects to plant secondary compounds. In: Rosenthal GA, Berenbaum MR (eds) Herbivores. their interactions with secondary plant metabolites. Academic Press, New York pp 45–88
Stamp NE (1990) Growth versus molting time of caterpillars as a function of temperature, nutrient concentration and the phenolic rutin. Oecologia 82:107–113
Stamp NE (1994) Simultaneous effects of potassium, rutin and temperature on performance of Manduca sexta caterpillars. Entomol Exp Appl 72:135–144
Thimann KV (1980) The senescence of leaves. In: Thimann KV (ed) Senescence in plants. CRC Press, Boca Raton pp 85–115
Thompson SN, Redak RA (2005) Feeding behaviour and nutrient selection in an insect Manduca sexta L., and alterations induced by parasitism. J Comp Physiol A 191:909–923
Thompson SN, Redak RA, Wang L-W (2001) Altered dietary nutrient intake maintains metabolic homeostasis in parasitized larvae of the insect Manduca sexta L. J Exp Biol 204:40656–4080
Thompson SN, Redak RA, Wang L-W (2005) Nutrition interacts with parasitism to influence growth and physiology of the insect Manduca sexta L. J Exp Biol 208:611–623
Trimmer BA, Weeks JC (1989) Effects of nicotinic and muscarinic agents on an identified motoneurone and its direct afferent inputs in larvae Manduca sexta. J Exp Biol 144:303–337
Tso TC (1972) Pysiology and biochemistry of tobacco plants. Dowden, Hutchinson and Ross, Stroudsburg
Vittorio PV, Krotkov G, Reed GB (1954) Incorporation of C14 into various carbohydrates of tobacco leaves after different periods of photosynthesis in C14O2. Science 119:906–908
Waldbauer GP, Friedman S (1991) Self-selection of optimal diets by insects. Annu Rev Entomol 36:43–63
Weseloh RM (1993) Potential effects of parasitoids on the evolution of caterpillar foraging behavior. In: Stamp NE, Casey TM (eds) Caterpillars: ecological and evolutionary constraints on foraging. Chapman and Hall, New York pp 203–223
Wink M, Theile V (2002) Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae). Chemoecology 12:29–46
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Thompson, S.N., Redak, R.A. Nicotine moderates the effects of macronutrient balance on nutrient intake by parasitized Manduca sexta L. J Comp Physiol B 177, 375–391 (2007). https://doi.org/10.1007/s00360-006-0136-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-006-0136-1