Abstract
Purpose
Current clinical prognostic factors are not accurate enough to identify and monitor those muscle-invasive bladder cancer (MIBC) patients at high risk of progression after radical cystectomy (RC). Here, we determined genetic alterations in the tumor and circulating tumor cell (CTC) enumeration to find biomarkers useful for the management of MIBC after RC.
Methods
Thirty-nine MIBC patients undergoing RC were included. Tumoral tissue DNA was analyzed by next generation sequencing. CTCs were isolated from blood collected before RC and one, four and 12 months later.
Results
Sixteen (41%) patients progressed in a median time of 8.5 months and 11 (69%) of these patients harbored the TERT c.-124C > T mutation. All progressive patients harboring the TERT c.-124C > T mutation presented a significant increase in CTC number 12 months after RC compared to those without the mutation. Additionally, CTC number at 12 months was identified as an independent prognostic biomarker for tumor progression and cancer specific survival (CSS). Ten (63%) progressive patients showed an increment of CTC number with a median anticipation period of four months compared with imaging techniques.
Conclusions
The TERT c.-124C > T mutation could be considered a biomarker of aggressivity. CTC enumeration is a useful tool for identifying MIBC patients at high risk of progression and CSS after RC and for detecting tumor progression earlier than imaging techniques.
Similar content being viewed by others
Introduction
Approximately 25% of bladder cancers (BC) are diagnosed as muscle-invasive bladder cancer (MIBC). Radical cystectomy (RC) with lymphadenectomy is the standard treatment for localized MIBC. However, despite undergoing radical surgery, around 50% of MIBC patients will develop local relapse or distant metastasis within two years of RC [1]. The current classification of MIBC is unable to individually predict which patients will relapse and develop metastases and when these events will occur during patient follow-up.
MIBC is an aggressive tumor with a high genetic instability and mutation rate in genes involved in transcription, chromatin regulation and the cell cycle [2]. The most common event described in BC to date is point mutations of the telomerase reverse transcriptase (TERT) promoter, present in approximately 80% of tumors, regardless of grade and stage [3]. However, due to the genetic heterogeneity of BC, there is no predictive or prognostic molecular information for its clinical application.
On the other hand, liquid biopsy has been recently used instead of tumor tissue to explore diagnostic, prognostic and predictive biomarkers in several tumors, including bladder cancer [4,5,6,7]. It is believed that circulating tumor cells (CTCs) present in liquid biopsy represent metastatic precursors that have an important role in disease invasion and progression [5]. In fact, several studies demonstrated that CTC number in peripheral blood correlates with poor outcome [5,6,7,8].
Both tumor tissue and liquid biopsy represent a source of prognostic biomarkers that could have an impact on MIBC patient management. Here, we evaluated DNA mutations in tumor samples from patients who underwent RC and enumerated the CTCs in their blood samples at different time points during their follow-up after RC to identify biomarkers predicting a high risk of recurrence and progression.
Materials and methods
Patients and samples
A total of 39 consecutive MIBC patients [median age (range) 70 years (51–85); 31 males, 8 females] who underwent RC and extended lymphadenectomy between 2018 and 2019 at our center were prospectively included. The clinicopathological features of the patients enrolled are summarized in Supplementary Table S1. Follow-up data was available for all patients (Supplementary Methods).
In our cohort, 8 of the 39 MIBC patients received neoadjuvant chemotherapy (NAC), and the remaining 31 MIBC patients did not for the following reasons: 11 patients were unfit for the NAC criteria, and 20 patients could not delay cystectomy.
Tissue samples were obtained from cystectomy specimens (N = 31). In case of pT0 in the cystectomy specimen, samples were obtained from transurethral resection of bladder tumor (TURBT) that showed muscle-invasive disease (N = 7). A tissue sample was not available for one patient (Patient 10) in the tumor biobank.
One 10 mL EDTA tube of peripheral blood was collected before RC and at one, four and 12 months after surgery. Blood samples were stored at room temperature until processed within the following 24 h.
Next generation sequencing (NGS) of tumoral tissue
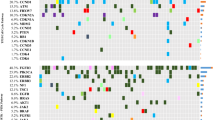
A total of 38 tissue samples were analyzed by NGS using the Ion Torrent Oncomine Comprehensive Assay v3 (Thermo Fisher Scientific, Massachusetts, USA) (Supplementary Methods and Supplementary Table S2). NGS data was analyzed with Ion Reporter Software v5.12 (Thermo Fisher Scientific) using a Oncomine Extended (5.18) filter to identify pathological mutations. Statistical analysis is detailed in Supplementary Methods.
CTC isolation and enumeration
CTCs from blood samples were isolated via the IsoFlux system (Fluxion, Biosciences) and stored at 4ºC until enumeration within the following two weeks (Supplementary Methods). CTCs were fixed and immunofluorescence stained using the CTC Enumeration Kit (Fluxion, Biosciences), following manufacturer’s instructions. CTC enumeration was performed manually using fluorescence microscopy (Supplementary Methods and Supplementary Figure S1). Molecular progression during follow-up was defined as the increase of at least 10 CTCs per 7.5 mL blood between two follow-up points. Statistical analysis is detailed in Supplementary Methods.
Results
Clinicopathological features of the cohort
Four of the 39 patients included in this study presented positive lymph nodes (LN +) at MIBC diagnosis (Supplementary Table S1). During a median follow-up of 25.5 months, 16 (41%) patients progressed (two with LN +). The median time to progression was 8.5 months (range 1–16 months). Overall, five of the 16 patients who progressed received neoadjuvant or adjuvant chemotherapy (one < pT2 and four pT3-4). The remaining seven pT3-4 patients had comorbidities or did not consent to neoadjuvant or adjuvant chemotherapy. Of the progressive patients, 81.3% (13/16) received treatment upon tumor progression. During follow-up, 15 (38.5%) patients died; 12 (80%) due to MIBC. Overall, 6.7% of the patients who died had LN + . Eight of the 12 (67%) patients who died had pT3 and pT4 tumors at MIBC diagnosis. The median time of cancer specific survival (CSS) was 15 months (range 4–31 months).
Four non-progressive patients developed another primary tumor during follow-up. These tumors were sarcoma, upper tract urothelial carcinoma, lung and ovarian cancers.
Mutations in tumor samples
A median of five (range 1–34) mutations were identified in bladder tumor samples (Supplementary Table S3). ATM, TP53 and TERT were the most frequently mutated genes in our cohort. Notably, the c.-124C > T hotspot mutation in the TERT promoter was found in 66% (25/38) of cases. Another TERT promoter mutation (c.-146C > T), the ATM c.1236-2A > T mutation and the TP53 c.853G > A mutation were found in 16%, 42% and 13% of samples, respectively. Interestingly, 92% of MIBC samples analyzed had at least one of these four mutations. Mean variant allele frequency (VAF) of TERT c.-124C > T, TERT c.-146C > T, ATM c.1236-2A > T and TP53 c.853G > A mutations was 0.17, 0.06, 0.05 and 0.06, respectively. Overall, a statistically significant correlation between high VAF (higher than the mean) and tumor progression was found for the TERT c.-124C > T mutation (p = 0.019).
Strikingly, 11 out of 16 (69%) progressive patients harbored the TERT c.-124C > T mutation, with all 11 patients having a high VAF.
CTC number for patient monitoring
CTCs were observed in all patients at time of cystectomy and during follow-up. Overall, the mean CTC number (range) at time of cystectomy and one, four and 12 months after RC was 35 (11–108), 35 (18–104), 33 (15–56) and 46 (12–132) CTCs per 7.5 mL blood, respectively.
CTC enumeration results and clinical variables during patient follow-up are summarized in Supplementary Figure S2. CTC number was significantly higher in progressive than in non-progressive patients 12 months after RC (p = 0.021) (Fig. 1A); however, no statistically significant differences were observed in CTC number between both groups of patients, neither at time of cystectomy (p = 0.855), nor one and four months after RC (p = 0.228; p = 0.191; respectively).
CTC enumeration in MIBC monitoring. Mean CTC number is shown at four different time points A for progressive and non-progressive MIBC patients and B for MIBC patients who underwent adjuvant chemotherapy (patients with CTC number ≥ 52 CTCs per 7.5 mL blood are considered at high risk of progression). C Time of progression according to CTC number (molecular progression) and imaging techniques (radiological progression). Molecular progression is considered when an increase of at least 10 CTCs per 7.5 mL blood is observed between two consecutive time points. Pt patient
Six patients from our cohort received adjuvant chemotherapy between two and four months after RC (Fig. 1B). According to CTC number, three of these patients were at high risk (≥ 52 CTCs per 7.5 mL blood; see “survival analysis”) of developing metastasis 12 months after RC. In fact, one patient (Pt 26) had a very high CTC number (121 CTCs per 7.5 mL blood) and presented clinical progression at 16 months after cystectomy. The other two patients (Pt 33 and Pt 39) had a CTC number close to the cut-off (56 and 60 CTCs per 7.5 mL blood); after a median follow-up of 24 months they did not show clinical progression. The three patients with a CTC number below cut-off did not present clinical progression after a median follow-up of 28 months.
Overall, 63% (10/16) of relapses were predicted earlier with CTC enumeration than with imaging techniques, whereas two cases were detected simultaneously by both techniques, and, in four cases, relapse was predicted earlier by imaging techniques. In two of these four cases we were unable to collect blood samples 12 months after cystectomy due to the Covid-19 pandemic, the samples were collected later. These two patients progressed 12 months after RC, but the increase in CTCs was observed at 14 and 15 months after RC, although we believe that the CTC increase would have been observed at 12 months, as the increase in CTC number from the last time point evaluated was greater than 40 CTCs per 7.5 mL blood.
Remarkably, an increase in CTC number predicted tumor relapse with a median anticipation period over conventional imaging techniques of 120 days versus 255 days (lead time, 4 months), respectively (p = 0.001) (Fig. 1C).
Correlation between tumor mutations and CTC enumeration
This study evaluated whether a relationship exists between gene mutation VAF and CTC enumeration after RC and at several follow-up time points. Overall, we found that MIBC patients harboring high VAF of the TERT c.-124c > T mutation (N = 14) presented a CTC number above the cut-off (≥ 52 CTCs per 7.5 mL blood; see “survival analysis”) 12 months after RC (p = 0.03). The remaining alterations (TERT c.-146C > T, TP53 c. 853G > A and ATM c.1236-2A > T) had no significant correlation with CTC enumeration.
Notably, progressive patients harboring the TERT c.-124C > T mutation (69%; 11/16) presented a significant increase in CTC number 12 months after RC compared with those progressive patients without a mutation (p = 0.014).
Survival analysis
Tumor tissue DNA mutations, CTC enumeration at each follow-up time point and clinical variables (pathological stage, lymph node status, chemotherapy) were evaluated by Cox regression analysis. Univariate analysis demonstrated that CTC number 12 months after RC and pathological stage are significant predictors of tumor progression. CTC number 12 months after RC and VAF of the TERT c.-124C > T mutation were significant predictors of cancer specific survival (CSS). Multivariate Cox regression analysis, including VAF of the TERT c.-124C > T mutation, CTC number at 12 months and pathological stage as covariables, identified CTC number 12 months after RC as an independent prognostic biomarker for tumor progression (HR 1.020; p = 0.009) and CSS (HR 1.021; p = 0.021).
The mean CTC number 12 months after RC (≥ 52 CTCs per 7.5 mL blood) was used as a cut-off point to classify patients into high- and low-risk groups for tumor progression and CSS. Figure 2 shows the Kaplan–Meier curves generated using the selected cut-off point, showing that this cut-off point can discriminate between two groups of MIBC patients with a significantly different probability of tumor progression and CSS.
Discussion
In recent years, the molecular characterization of BC has considerably advanced our understanding of BC tumor biology; however, to date, there is no prognostic biomarker established in clinical practice to identify and monitor those MIBC patients at high risk of progression after RC. To the best of our knowledge, this is the first study to evaluate DNA mutations in MIBC, enumerate blood CTCs during follow-up of patients after RC and to combine them to identify prognostic biomarkers and improve MIBC management.
In our cohort of MIBC patients, the most frequently found mutations in tumors were TERT c.-124C > T, TERT c.-146C > T, ATM c.1236-2A > T and TP53 c.853G > A. TERT promoter mutations c.-124C > T and c.-146C > T have been frequently described in BC [9, 10] and several other tumors [11, 12]. TERT mutations have been detected in all stages of BC and distant metastasis, suggesting an important role of telomerase activity in the tumorigenesis of BC [9]. Mutations in the TP53 and ATM genes have also been previously described in BC [13], although, as far as we know, the two specific mutations found in our cohort have never been described in BC. Of note, the TP53 c.853G > A mutation has been found as a germline mutation in breast and lung tumors [14, 15] associated with an increased risk of cancer. Interestingly, at least one of these four mutations were present in 92% of MIBC samples, with TERT c.-124C > T being the most frequent (66% of patients). Furthermore, a high VAF for the TERT c.-124C > T mutation correlates with tumor progression. Of note, this mutation was overrepresented in the subset of progressive compared with non-progressive patients, showing a high VAF in all progressive patients. According to our results, the TERT c.-124C > T mutation has been previously described as a biomarker of recurrence and progression in BC [9, 10, 16]. Overall, these findings suggest that the presence of the TERT c.-124C > T mutation could play a crucial role in the aggressiveness of BC.
In the present study, we also found that progressive MIBC patients presented a significant increase in the mean CTC number 12 months after RC. It has been described that CTC number increases with the higher tumor burden [17,18,19]. Thus, CTC number could be used as a biomarker of tumor burden during follow-up of MIBC patients. Furthermore, CTCs number 12 months after RC was able to distinguish two groups of patients with a different probability of progression and CSS. A comparable result was obtained by Rink et al. [20] and Zhang et al. [21] who concluded that CTC number is a predictor of tumor progression and CSS in MIBC.
Interestingly, we showed that CTC enumeration could be a useful tool to monitor tumor relapse after systemic therapy. This result should be taken with caution, since the subset of patients from our cohort who underwent adjuvant chemotherapy is limited; however, other authors previously reported that CTC enumeration was useful for evaluating the efficacy of treatments in cancer patients [22, 23].
On the other hand, we observed a significant increase in CTC number a median of four months before radiological progression in 63% of progressive patients. A similar result was found by Christensen et al. [24] but by analyzing circulating tumor DNA instead of CTCs in a series of 99 MIBC patients. This result emphasizes the utilization of liquid biopsy for early detection of tumor progression during MIBC patient follow-up.
Finally, the correlation between mutation analyses and CTC enumeration indicated that progressive patients with a high VAF of the TERT c.-124C > T mutation presented a CTC number exceeding the cut-off 12 months after RC, reinforcing the fact that the TERT c.-124C > T mutation could be considered a biomarker of aggressivity in BC.
The relevance of the present work lies in the fact that it is the first to combine tumor mutational analysis with CTC enumeration at different time points during follow-up of MIBC patients after RC. Our results suggest that those patients harboring tumors with high VAF of the TERT c.-124C > T mutation are at higher risk of progression and, therefore, could be optimal candidates for closer follow-up via CTC enumeration. It is important to note that the methodology used to analyze mutations and CTCs is easily available in diagnostic laboratories, reasonably simple and has a higher sensitivity than other technologies such as the FDA approved CellSearch system [22, 25,26,27] and, thus, it can be easily implemented in clinical practice. However, we must acknowledge some study limitations, such as the high economic cost of isolating CTCs, the series size (although our series has been represented by a total of 150 samples analyzed) and the low number of patients who received chemotherapy. A final validation of our results in a larger and independent series is necessary to define the real role of the TERT c.-124C > T mutation and CTC enumeration as prognostic biomarkers in MIBC patients after RC.
Conclusion
The presence of the TERT c.-124C > T mutation in muscle-invasive bladder tumor could be considered a biomarker of tumor aggressivity. CTC enumeration in the bloodstream of MIBC patients after RC is a useful tool to identify patients with a high risk of progression and CSS during follow-up and to detect tumor progression earlier than imaging techniques. MIBC patients harboring a high VAF of the TERT c.-124C > T mutation and high CTC number in the follow-up had a greater risk of progression. The implementation of this combined non-invasive tool in the clinical setting could have an impact on disease management since patients could benefit from early treatment.
Abbreviations
- BC:
-
Bladder cancer
- CSS:
-
Cancer specific survival
- CTC:
-
Circulating tumor cell
- ctDNA:
-
Circulating tumor DNA
- EMT:
-
Epithelial-mesenchymal transition
- LN:
-
Lymph nodes
- MIBC:
-
Muscle-invasive bladder cancer
- NAC:
-
Neoadjuvant chemotherapy
- NGS:
-
Next generation sequencing
- PBMC:
-
Peripheral blood mononuclear cell
- RC:
-
Radical cystectomy
- TERT:
-
Telomerase reverse transcriptase
- TURBT:
-
Transurethral resection of bladder tumor
- VAF:
-
Variant allele frequency
References
Witjes JA, Bruins HM, Cathomas R et al (2021) European Association of Urology Guidelines on muscle invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 79:82–104. https://doi.org/10.1016/j.eururo.2020.03.055
Kim J, Akbani R, Creighton CJ et al (2015) Invasive bladder cancer: genomic insights and therapeutic promise. Clin Cancer Res 21:4514–4524. https://doi.org/10.1158/1078-0432.CCR-14-1215
Ma K, Hurst CD (2015) Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 15:25–41. https://doi.org/10.1038/nrc3817
Nagata M, Muto S, Horie S (2016) Molecular biomarkers in bladder cancer: novel potential indicators of prognosis and treatment outcomes. Dis Markers 2016:8205836. https://doi.org/10.1155/2016/8205836
Alix-Panabières C, Pantel K (2016) Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 6:479–491. https://doi.org/10.1158/2159-8290.CD-15-1483
Luo W, Rao M, Qu J, Luo D (2018) Applications of liquid biopsy in lung cancer-diagnosis, prognosis prediction, and disease monitoring. Am J Transl Res 10:3911–3923
Nicolazzo C, Busetto GM, Gradilone A et al (2019) Circulating tumor cells identify patients with super-high-risk non-muscle-invasive bladder cancer: updated outcome analysis of a prospective single-center trial. Oncologist 24:612–616. https://doi.org/10.1634/theoncologist.2018-0784
Bidard F-C, Michiels S, Riethdorf S et al (2018) Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst 110:560–567. https://doi.org/10.1093/jnci/djy018
Günes C, Wezel F, Southgate J, Bolenz C (2018) Implications of TERT promoter mutations and telomerase activity in urothelial carcinogenesis. Nat Rev Urol 15:386–393. https://doi.org/10.1038/s41585-018-0001-5
Leão R, Lee D, Figueiredo A et al (2019) Combined genetic and epigenetic alterations of the TERT promoter affect clinical and biological behavior of bladder cancer. Int J Cancer 144:1676–1684. https://doi.org/10.1002/ijc.31935
Barthel FP, Wei W, Tang M et al (2017) Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet 49:349–357. https://doi.org/10.1038/ng.3781
Vinagre J, Almeida A, Pópulo H et al (2013) Frequency of TERT promoter mutations in human cancers. Nat Commun 4:2185. https://doi.org/10.1038/ncomms3185
Pan Y-H, Zhang J-X, Chen X, Liu F et al (2021) Predictive value of the TP53/PIK3CA/ATM mutation classifier for patients with bladder cancer responding to immune checkpoint inhibitor therapy. Front Immunol 12:643282. https://doi.org/10.3389/fimmu.2021.643282
Lee DSC, Yoon S-Y, Looi LM et al (2012) Comparable frequency of BRCA1, BRCA2 and TP53 germline mutations in a multi-ethnic Asian cohort suggests TP53 screening should be offered together with BRCA1/2 screening to early-onset breast cancer patients. Breast Cancer Res 14:R66. https://doi.org/10.1186/bcr3172
Yang D, Han X, Li D et al (2020) Molecular diagnosis and clinical outcome of a lung cancer patient with TP53-E285K mutated Li-Fraumeni syndrome harboring a somatic EGFR-KDD mutation. Am J Transl Res 12:6689–6693
Hayashi Y, Fujita K, Nojima S et al (2020) TERT C228T mutation in non-malignant bladder urothelium is associated with intravesical recurrence for patients with non-muscle invasive bladder cancer. Mol Oncol 14:2375–2383. https://doi.org/10.1002/1878-0261.12746
Gallagher DJ, Milowsky MI, Ishill N, Trout A et al (2009) Detection of circulating tumor cells in patients with urothelial cancer. Ann Oncol 20:305–308. https://doi.org/10.1093/annonc/mdn627
Naoe M, Ogawa Y, Morita J et al (2007) Detection of circulating urothelial cancer cells in the blood using the cell search system. Cancer 109:1439–1445. https://doi.org/10.1002/cncr.22543
Rink M, Chun FKH, Minner S et al (2011) Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU Int 107:1668–1675. https://doi.org/10.1111/j.1464-410X.2010.09562.x
Rink M, Chun FK, Dahlem R et al (2012) Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy : a prospective study. Eur Urol 61:810–817. https://doi.org/10.1016/j.eururo.2012.01.017
Zhang Z, Fan W, Deng Q et al (2017) The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: a meta- analysis of 30 published studies. Oncotarget 8:59527–59538. https://doi.org/10.18632/oncotarget.18521
Alva A, Friendlancer T, Clark M et al (2015) Circulating tumor cells as potential biomarkers in bladder cancer. J Urol 194:790–798. https://doi.org/10.1016/j.juro.2015.02.2951
Lin M, Liang S-Z, Shi J et al (2017) Circulating tumor cell as a biomarker for evaluating allogenic NK cell immunotherapy on stage IV non-small cell lung cancer. Immunol Lett 191:10–15. https://doi.org/10.1016/j.imlet.2017.09.004
Christensen E, Birkenkamp-Demtröder K, Sethi H et al (2019) Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 37:1547–1557. https://doi.org/10.1200/JCO.18.02052
Nimir M, Ma Y, Jeffreys SA et al (2019) Detection of AR-V7 in liquid biopsies of castrate resistant prostate cancer patients: a comparison of AR-V7 analysis in circulating tumor cells. Circ Tumor RNA Exosomes Cells 8:688. https://doi.org/10.3390/cells8070688
Ding PN, Becker TM, Bray VJ et al (2019) The predictive and prognostic significance of liquid biopsy in advanced epidermal growth factor receptor-mutated non-small cell lung cancer: a prospective study. Lung Cancer 134:187–193. https://doi.org/10.1016/j.lungcan.2019.06.021
Toh JWT, Lim SH, MacKenzie S et al (2020) Association between microsatellite instability status and peri-operative release of circulating tumour cells in colorectal cancer. Cells 9:425. https://doi.org/10.3390/cells9020425
Acknowledgements
We thank all the patients who participated in this study and all the staff and nurses of the Urology Department of the Hospital Clinic for collaborating with sample collection. We are indebted to the IDIBAPS biobank for tissue sample procurement. We thank Helena Kruyer for the English correction of the manuscript. Funding from CERCA Programme/Generalitat de Catalunya is gratefully acknowledged. This work was developed at the building Centre de Recerca Biomèdica Cellex, Barcelona. All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the Instituto de Salud Carlos III (ISCIII) through the Plan Estatal de Investigación Científica y Técnica y de Innovación 2018–2020, project reference number PI17/01343 and co-funded by the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Contributions
LI and LM designed the study and obtained the funding; RC, MIT and AG performed the experiments; RC performed the statistical analysis; FLR and NS took part in the study; RC, LM and LI wrote the paper; MJR, LM, LI and AA supervised the study. The manuscript has been reviewed and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Ethics approval
The study was approved by the Clinical Research Ethics Committee of Hospital Clinic of Barcelona (HCB/2018/0026). All methods were carried out in accordance with relevant guidelines and regulations.
Consent to participate
Informed consent (HCB/2013/8753) was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carrasco, R., Ingelmo-Torres, M., Gómez, A. et al. Prognostic implication of TERT promoter mutation and circulating tumor cells in muscle-invasive bladder cancer. World J Urol 40, 2033–2039 (2022). https://doi.org/10.1007/s00345-022-04061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-04061-9